Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R0FO
|
|||
| Former ID |
DCL000786
|
|||
| Drug Name |
Lenvatinib
|
|||
| Synonyms |
E 7080; E-7080, E7080; 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Thyroid cancer [ICD-11: 2D10; ICD-10: C73] | Approved | [1] | |
| Hepatocellular carcinoma [ICD-11: 2C12.02; ICD-10: C22.0; ICD-9: 155] | Phase 3 | [2] | ||
| Renal cell carcinoma [ICD-11: 2C90; ICD-10: C64; ICD-9: 189] | Phase 3 | [3] | ||
| Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 2 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1/2 | [3] | ||
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 1 | [4] | ||
| Non-small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162] | Application submitted | [3] | ||
| Company |
Eisai Co. Ltd.
|
|||
| Structure |
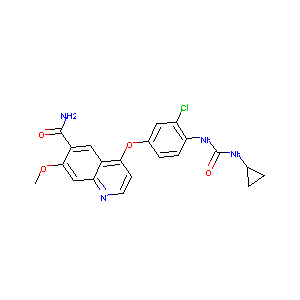 |
Download2D MOL |
||
| Formula |
C21H19ClN4O4
|
|||
| Canonical SMILES |
COC1=CC2=NC=CC(=C2C=C1C(=O)N)OC3=CC(=C(C=C3)NC(=O)NC4CC4)Cl
|
|||
| InChI |
1S/C21H19ClN4O4/c1-29-19-10-17-13(9-14(19)20(23)27)18(6-7-24-17)30-12-4-5-16(15(22)8-12)26-21(28)25-11-2-3-11/h4-11H,2-3H2,1H3,(H2,23,27)(H2,25,26,28)
|
|||
| InChIKey |
WOSKHXYHFSIKNG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 417716-92-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14782907, 24126764, 44859775, 74753053, 99436922, 123098996, 123110209, 124757043, 125163847, 125749042, 126665901, 131314303, 131480711, 134221774, 135262444, 135626656, 135685148, 135685149, 135685168, 136367339, 136367958, 137262627, 137276042, 139802275, 144115929, 152258284, 152344144, 160647123, 162011787, 162037768, 162527769, 164041901, 174560999, 178103998, 180386840, 198978519, 202553041, 223669921, 223705252, 223913134, 227134592, 242060265, 247802696, 251911433, 251971223, 252150297, 252215326, 252215327, 252451828, 252543308
|
|||
| ChEBI ID |
CHEBI:85994
|
|||
| ADReCS Drug ID | BADD_D01254 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2018. Application Number: (ANDA) 208627. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs. 2008 Sep;13(3):523-36. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

