Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R4UW
|
|||
| Former ID |
DCL000964
|
|||
| Drug Name |
Roflumilast
|
|||
| Synonyms |
162401-32-3; DAXAS; Daliresp; 3-(CYCLOPROPYLMETHOXY)-N-(3,5-DICHLOROPYRIDIN-4-YL)-4-(DIFLUOROMETHOXY)BENZAMIDE; BY217; BYK20869; UNII-0P6C6ZOP5U; BY-217; Roflumilast (Daxas); B9302-107; 0P6C6ZOP5U; 3-(Cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide; Benzamide, 3-(cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)-; CHEMBL193240; CHEBI:47657; BYK-20869; ROF; Libertek; AK110425; 3-Cyclopropylmethoxy-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide; DAXAS; Roflumilast [USAN]; APTA-2217; Roflumilast (JAN/USAN/INN); 3-cyclopropylmethoxy-4-difluoromethoxy-N-(3,5-di-chloropyrid-4-yl)benzamide; Alogliptin/roflumilast
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Approved | [1], [2] | |
| Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 1 | [3] | ||
| Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Withdrawn from market | [1], [4] | ||
| Atopic dermatitis [ICD-11: EA80; ICD-10: L20; ICD-9: 691.8, 692.9] | Discontinued in Phase 2 | [1], [4] | ||
| Company |
Nycomed US Inc; Altana
|
|||
| Structure |
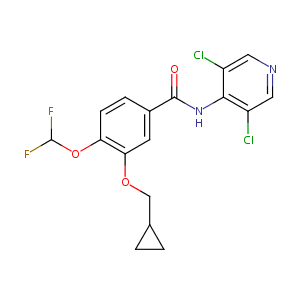 |
Download2D MOL |
||
| Formula |
C17H14Cl2F2N2O3
|
|||
| Canonical SMILES |
C1CC1COC2=C(C=CC(=C2)C(=O)NC3=C(C=NC=C3Cl)Cl)OC(F)F
|
|||
| InChI |
1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24)
|
|||
| InChIKey |
MNDBXUUTURYVHR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 162401-32-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7890296, 10300323, 12014977, 14879303, 36890842, 46393569, 46393577, 46515099, 47207405, 49876227, 50659842, 53788863, 57288829, 57404909, 58108277, 85239858, 87235814, 103463298, 104253463, 104640579, 109693509, 121279025, 124757594, 124772083, 125001902, 125164398, 125536640, 126618387, 126666054, 126731090, 128619725, 134339138, 134339348, 134339400, 134358320, 135114471, 136345716, 136946527, 137010091, 137269493, 142175980, 144223762, 160644734, 162011650, 162037767, 162201204, 163124095, 163406034, 163620892, 163686213
|
|||
| ChEBI ID |
CHEBI:47657
|
|||
| ADReCS Drug ID | BADD_D01962 | |||
| SuperDrug ATC ID |
R03DX07
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Ruminococcus gnavus ATCC 29149
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Description | Roflumilast can be metabolized by Ruminococcus gnavus ATCC 29149. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Lactococcus lactis subsp. lactis Il1403
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Description | Roflumilast can be accumulated by Lactococcus lactis subsp. lactis Il1403. | |||
|
Studied Microbe: Streptococcus salivarius
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Description | Roflumilast can be metabolized by Streptococcus salivarius. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 4 (PDE4) | Target Info | Modulator | [2], [6] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6962). | |||
| REF 2 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||
| REF 3 | ClinicalTrials.gov (NCT01664624) Roflumilast Plus Alogliptin Proof-of-Mechanism Study in Type2 Diabetes. U.S. National Institutes of Health. | |||
| REF 4 | Emerging drugs for atopic dermatitis. Expert Opin Emerg Drugs. 2009 Mar;14(1):165-79. | |||
| REF 5 | Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. 2021 Sep;597(7877):533-538. | |||
| REF 6 | Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease.Br J Pharmacol.2011 May;163(1):53-67. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

