Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S5LH
|
|||
| Former ID |
DAP000562
|
|||
| Drug Name |
Edrophonium
|
|||
| Synonyms |
EDR; Edrophonum; Edroponium; Reversol; Tensilon; EDROPHONIUM ION; Edrophone Chloride; Enlon Plus; ENLON-PLUS; Enlon (TN); Reversol (TN); Tensilon (TN); Ethyl-(3-hydroxyphenyl)-dimethylazanium; Ethyl-(3-hydroxy-phenyl)-dimethyl-ammonium; N-ethyl-3-hydroxy-N,N-dimethylanilinium; N-ethyl-3-hydroxy-N,N-dimethylbenzenaminium; (3-hydroxyphenyl)dimethylethylammonium; 3-hydroxy-N,N-dimethyl-N-ethylanilinium
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Myasthenia gravis [ICD-11: 8C6Y; ICD-9: 358] | Approved | [1] | |
| Therapeutic Class |
Antidotes
|
|||
| Structure |
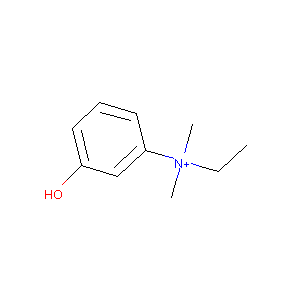 |
Download2D MOL |
||
| Formula |
C10H16NO+
|
|||
| Canonical SMILES |
CC[N+](C)(C)C1=CC(=CC=C1)O
|
|||
| InChI |
1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1
|
|||
| InChIKey |
VWLHWLSRQJQWRG-UHFFFAOYSA-O
|
|||
| CAS Number |
CAS 312-48-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9190, 6212622, 7366196, 7887282, 7979147, 8152037, 11111143, 11111144, 11335984, 11361223, 11363336, 11365898, 11368460, 11374171, 11376622, 11462195, 11466111, 11467231, 11484956, 11485681, 11488926, 11492551, 11494256, 26702965, 26713721, 29222344, 46507530, 47365233, 47365234, 47515350, 47589038, 47662326, 47810792, 48035164, 48110488, 49698977, 50064671, 50100235, 50104222, 50923308, 53788287, 57321660, 58106942, 87350897, 90341680, 103812420, 103951705, 104234195, 104302825, 117617165
|
|||
| ChEBI ID |
CHEBI:251408
|
|||
| ADReCS Drug ID | BADD_D00747 ; BADD_D00748 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Acetylcholinesterase (AChE) | Target Info | Inhibitor | [2], [3], [4] |
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Cholinergic synapse | ||||
| Panther Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | ||||
| Pathwhiz Pathway | Phospholipid Biosynthesis | |||
| Pathway Interaction Database | ATF-2 transcription factor network | |||
| WikiPathways | Monoamine Transport | |||
| Biogenic Amine Synthesis | ||||
| Acetylcholine Synthesis | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040131. | |||
| REF 2 | Screening of acetylcholinesterase inhibitors by CE after enzymatic reaction at capillary inlet. J Sep Sci. 2009 May;32(10):1748-56. | |||
| REF 3 | Neuromuscular blockade, reversal agent use, and operating room time: retrospective analysis of US inpatient surgeries. Curr Med Res Opin. 2009 Apr;25(4):943-50. | |||
| REF 4 | Stabilization of Torpedo californica acetylcholinesterase by reversible inhibitors. Biochemistry. 2009 Jan 27;48(3):563-74. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

