Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T8GD
|
|||
| Former ID |
DAP000175
|
|||
| Drug Name |
Nicotine
|
|||
| Synonyms |
Habitrol; Micotine; NCT; NICOTINE AND SALTS; Nicoderm Patch; Nicotina [Italian]; Nicotine Patch; Nicotine [USAN]; Nicotine replacement patch; Nicotine solution; Nictoine patch; Nikotin [German]; Nikotyna [Polish]; Transdermal Nicotine; Nicotine [UN1654] [Poison]; ENT 3,424; Habitrol (TN); Nicorette (TN); Nicotine (USP); Nicotine (compounds related to); Nicotine [BSI:ISO]; L(-)-nicotine; Beta-Pyridyl-alpha-N-methyl pyrrolidine; (-)-Nicotine solution; (2S) 3-(1-Methyl-pyrrolidin-2-yl)-pyridine; (S)-(-)-NICOTINE, 3-[(2S)-1-METHYL-2-PYRROLIDINYL] PYRIDINE; (S)-(-)-Nicotine; (S)-3-(1-methylpyrrolidin-2-yl)pyridine; (S)-3-(N-methylpyrrolidin-2-yl)pyridine; (S)-Nicotine; 3-(2-(N-methylpyrrolidinyl))pyridine; 3-(N-Methylpyrollidino)pyridine; 3-(N-Methylpyrrolidino)pyridine; 3-[(2S)-1-methylpyrrolidin-2-yl]pyridine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Nicotine dependence [ICD-11: 6C4A.2; ICD-10: F17.2; ICD-9: 305.1] | Approved | [1], [2] | |
| Tobacco dependence [ICD-11: 6C4A.2; ICD-10: F17.1] | Clinical trial | [3] | ||
| Company |
Upjohn Corporation
|
|||
| Structure |
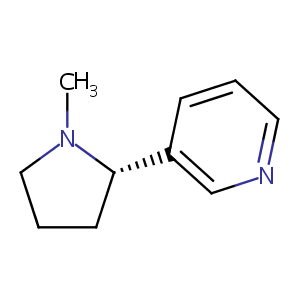 |
Download2D MOL |
||
| Formula |
C10H14N2
|
|||
| Canonical SMILES |
CN1CCCC1C2=CN=CC=C2
|
|||
| InChI |
1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1
|
|||
| InChIKey |
SNICXCGAKADSCV-JTQLQIEISA-N
|
|||
| CAS Number |
CAS 54-11-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4007, 794580, 829184, 829311, 833584, 3137564, 7889325, 7980122, 8144582, 10223753, 11537938, 14748051, 15171051, 17389805, 17397514, 24862741, 24870064, 24897663, 24897737, 25621940, 26752744, 26752745, 26752746, 44420693, 46393352, 47515453, 47515454, 47959911, 48110586, 48414101, 48423841, 48425389, 49854422, 50105392, 50105393, 50271161, 53788316, 53801040, 56310810, 56311904, 56312242, 56312459, 56312463, 56312785, 56313106, 56313907, 56314421, 56314547, 56314548, 56314824
|
|||
| ChEBI ID |
CHEBI:17688
|
|||
| ADReCS Drug ID | BADD_D01564 | |||
| SuperDrug ATC ID |
N07BA01
|
|||
| SuperDrug CAS ID |
cas=000054115
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Nicotinic acetylcholine receptor (nAChR) | Target Info | Antagonist | [4], [5] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2585). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 074611. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Atypical antipsychotics as noncompetitive inhibitors of alpha4beta2 and alpha7 neuronal nicotinic receptors. Neuropharmacology. 2009 Aug;57(2):183-91. | |||
| REF 5 | Development and optimization of a high-throughput electrophysiology assay for neuronal alpha4beta2 nicotinic receptors. J Neurosci Methods. 2009 Aug 30;182(1):17-24. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

