Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0YB1G
|
|||
| Former ID |
DIB004258
|
|||
| Drug Name |
Fentiazac
|
|||
| Synonyms |
FENTIAZAC; Donorest; Flogene; Norvedan; 18046-21-4; BR 700; UNII-0YHF6E6NLS; Fentiazacum [INN-Latin]; Fentiazaco [INN-Spanish]; 2-(4-(4-chlorophenyl)-2-phenylthiazol-5-yl)acetic acid; EINECS 241-958-1; CH 800; 0YHF6E6NLS; NSC 282191; C17H12ClNO2S; 4-(p-Chlorophenyl)-2-phenyl-5-thiazoleacetic acid; BRN 1083610; 5-Thiazoleacetic acid, 4-(p-chlorophenyl)-2-phenyl-; 5-Thiazoleacetic acid, 4-(4-chlorophenyl)-2-phenyl-; MLS003115545; WY 21,894; CHEMBL589092; 4-(4-Chlorophenyl)-2-phenyl-5-thiazoleacetic acid; NCGC00182976-01
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Dysmenorrhea [ICD-11: GA34.3; ICD-9: 625.3] | Approved | [1] | |
| Structure |
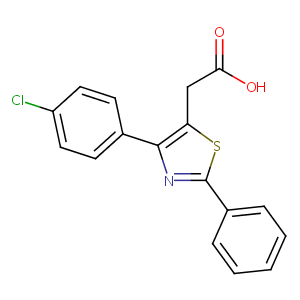 |
Download2D MOL |
||
| Formula |
C17H12ClNO2S
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C2=NC(=C(S2)CC(=O)O)C3=CC=C(C=C3)Cl
|
|||
| InChI |
1S/C17H12ClNO2S/c18-13-8-6-11(7-9-13)16-14(10-15(20)21)22-17(19-16)12-4-2-1-3-5-12/h1-9H,10H2,(H,20,21)
|
|||
| InChIKey |
JIEKMACRVQTPRC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 18046-21-4
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:94523
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Clostridioides difficile
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Clostridioides difficile was decreased by Fentiazac (adjusted p-values: 6.34E-04). | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

