Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00FHR
|
|||
| Former ID |
DCL001114
|
|||
| Drug Name |
MGCD-0103
|
|||
| Synonyms |
Mocetinostat; MG 0103; MG 4230; MG 4915; MG 5026; MG0103; MG4230; MG4915; MG5206; MGCD 0103; MGCD0103; MG-0103; MG-4230; MG-4915; MG-5026; Mocetinostat, MGCD0103; N-(2-aminophenyl)-4-[[(4-pyridin-3-ylpyrimidin-2-yl)amino]methyl]benzamide; N-(2-Aminophenyl)-4-((4-pyridin-3-ylpyrimidin-2-ylamino)methyl)benzamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Non-small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162] | Phase 2 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 2 | [2], [3] | ||
| Diffuse large B-cell lymphoma [ICD-11: 2A81; ICD-10: C83.3; ICD-9: 200] | Phase 1/2 | [4] | ||
| Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 1 | [5] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
MethylGene Inc
|
|||
| Structure |
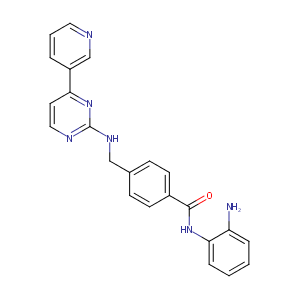 |
Download2D MOL |
||
| Formula |
C23H20N6O
|
|||
| Canonical SMILES |
C1=CC=C(C(=C1)N)NC(=O)C2=CC=C(C=C2)CNC3=NC=CC(=N3)C4=CN=CC=C4
|
|||
| InChI |
1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29)
|
|||
| InChIKey |
HRNLUBSXIHFDHP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 726169-73-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14830272, 24157526, 45287781, 56269575, 57373597, 75001461, 87226489, 87350368, 99436992, 103571737, 124490381, 124757017, 125163821, 125570876, 126671642, 131480763, 134964362, 135200342, 135723831, 136340181, 136367318, 136367876, 136920348, 137061752, 137276026, 142701494, 144116214, 152254622, 152258401, 152344187, 160647238, 162011468, 162037450, 162202711, 164042034, 164193968, 171578666, 172914178, 174007066, 174530499, 175608166, 177748388, 178103587, 186007035, 187071895, 188899510, 198936135, 218902997, 223387412, 223679945
|
|||
| ChEBI ID |
CHEBI:94525
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7008). | |||
| REF 3 | MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008 Apr;7(4):759-68. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 6 | Protein methyltransferases as a target class for drug discovery. Nat Rev Drug Discov. 2009 Sep;8(9):724-32. | |||
| REF 7 | Histone deacetylase inhibitors in cancer therapy: latest developments, trends and medicinal chemistry perspective. Anticancer Agents Med Chem. 2007 Sep;7(5):576-92. | |||
| REF 8 | Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006 Sep;5(9):769-84. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

