Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00HPK
|
|||
| Former ID |
DAP000752
|
|||
| Drug Name |
Naftifine
|
|||
| Synonyms |
Naftifin; Naftifina; Naftifinum; Naftifine HCl; Naftifina [INN-Spanish]; Naftifine (INN); Naftifine [INN:BAN]; Naftifinum[INN-Latin]; Naftin (TN); SN 105-843; N-cinnamyl-N-methyl-1-naphthalenemethylamine hydrochloride; N-Methyl-N-(1-naphthyl methyl)-3-phenyl-2-propen-1-amine(E), hydrochloride; (2E)-N-methyl-N-(1-naphthylmethyl)-3-phenyl-2-propen-1-amine; (E)-N-Cinnamyl-N-methyl-1-naphthalenemethylamine; (E)-N-Cinnamyl-N-methyl-1-naphthalinmethylamin; (E)-N-Cinnamyl-N-methyl-1-naphthylmethylamin; (E)-N-methyl-N-(naphthalen-1-ylmethyl)-3-phenylprop-2-en-1-amine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Dermatomycosis [ICD-11: EA60] | Approved | [1], [2] | |
| Therapeutic Class |
Antifungal Agents
|
|||
| Company |
Merz Pharmaceuticals Llc
|
|||
| Structure |
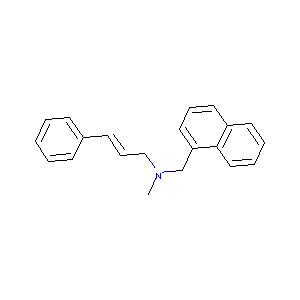 |
Download2D MOL |
||
| Formula |
C21H21N
|
|||
| Canonical SMILES |
CN(CC=CC1=CC=CC=C1)CC2=CC=CC3=CC=CC=C32
|
|||
| InChI |
1S/C21H21N/c1-22(16-8-11-18-9-3-2-4-10-18)17-20-14-7-13-19-12-5-6-15-21(19)20/h2-15H,16-17H2,1H3/b11-8+
|
|||
| InChIKey |
OZGNYLLQHRPOBR-DHZHZOJOSA-N
|
|||
| CAS Number |
CAS 65472-88-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10271, 603411, 7980063, 8180321, 14942609, 34712608, 47646744, 47720796, 48094744, 48169595, 49699388, 50065026, 50124043, 50962514, 85788086, 96024933, 103188450, 103840051, 104057027, 104353418, 117392353, 124766151, 126533983, 126629054, 126657705, 126685418, 134338038, 135029113, 137006423, 142192259, 144106432, 175266913, 177749226, 179150867, 179225939, 184546230, 196106120, 223440381, 223677934, 224441276, 226432968, 229686166, 251912313, 251916624, 252354411, 252817647
|
|||
| ChEBI ID |
CHEBI:7451
|
|||
| ADReCS Drug ID | BADD_D01517 ; BADD_D01518 | |||
| SuperDrug ATC ID |
D01AE22
|
|||
| SuperDrug CAS ID |
cas=065472880
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Squalene monooxygenase (SQLE) | Target Info | Inhibitor | [3], [4] |
| KEGG Pathway | Steroid biosynthesis | |||
| Sesquiterpenoid and triterpenoid biosynthesis | ||||
| Metabolic pathways | ||||
| Biosynthesis of secondary metabolites | ||||
| Biosynthesis of antibiotics | ||||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| Reactome | Cholesterol biosynthesis | |||
| Activation of gene expression by SREBF (SREBP) | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 019356. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | Characterization of squalene epoxidase activity from the dermatophyte Trichophyton rubrum and its inhibition by terbinafine and other antimycotic agents. Antimicrob Agents Chemother. 1996 Feb;40(2):443-7. | |||
| REF 4 | Mode of action of anti-Candida drugs: focus on terconazole and other ergosterol biosynthesis inhibitors. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 2):1193-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

