Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00TLM
|
|||
| Former ID |
DNC011099
|
|||
| Drug Name |
PRUVANSERIN
|
|||
| Synonyms |
Pruvanserin; UNII-UL09X1D9EM; 443144-26-1; UL09X1D9EM; CHEMBL1215661; Pruvanserin [USAN:INN]; 7-{4-(2-(4-Fluorophenyl)ethyl)piperazine-1-carbonyl}-1H-indole-3-carbonitrile; 7-[4-[2-(4-fluorophenyl)ethyl]piperazine-1-carbonyl]-1H-indole-3-carbonitrile; AC1O5FMC; Pruvanserin (USAN/INN); SCHEMBL678751; DTXSID40196133; AQRLDDAFYYAIJP-UHFFFAOYSA-N; ZINC56898757; BDBM50324540; LSN2422347; SB16534; EMD 390920; DB13094; EMD-390920; LSN-2422347; NCGC00370956-01; LY2420586; LY-2422347; D06632; L001679
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Sleep-wake disorder [ICD-11: 7A00-7B2Z; ICD-10: G47] | Phase 2 | [1] | |
| Structure |
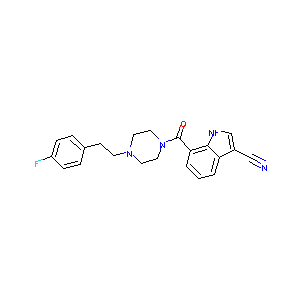 |
Download2D MOL |
||
| Formula |
C22H21FN4O
|
|||
| Canonical SMILES |
C1CN(CCN1CCC2=CC=C(C=C2)F)C(=O)C3=CC=CC4=C3NC=C4C#N
|
|||
| InChI |
1S/C22H21FN4O/c23-18-6-4-16(5-7-18)8-9-26-10-12-27(13-11-26)22(28)20-3-1-2-19-17(14-24)15-25-21(19)20/h1-7,15,25H,8-13H2
|
|||
| InChIKey |
AQRLDDAFYYAIJP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 443144-26-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 2A receptor (HTR2A) | Target Info | Modulator | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Gap junction | ||||
| Serotonergic synapse | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | 5HT2 type receptor mediated signaling pathway | |||
| Reactome | Serotonin receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Serotonin Receptor 2 and STAT3 Signaling | |||
| Serotonin Receptor 2 and ELK-SRF/GATA4 signaling | ||||
| SIDS Susceptibility Pathways | ||||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00259311) Efficacy Study of LY2422347 to Treat Insomnia. U.S. National Institutes of Health. | |||
| REF 2 | 5-HT(2A) inverse-agonists for the treatment of insomnia. Curr Top Med Chem. 2008;8(11):969-76. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

