Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00TLN
|
|||
| Former ID |
DAP000103
|
|||
| Drug Name |
Cyproheptadine
|
|||
| Synonyms |
Ciproheptadina; Ciprovit; Cypoheptadine; Cyproheptadiene; Cyproheptadinum; Dihexazin; Dronactin; Eiproheptadine; Periactin; Periactine; Periactinol; Viternum; Cyproheptadine Hcl; MK 141; Ciproheptadina [INN-Spanish]; Ciprovit (TN); Cyproheptadine (INN); Cyproheptadine [INN:BAN]; Cyproheptadinum [INN-Latin]; Dibenzosuberonone/Cyproheptadine; Periactin (TN); 1-Methyl-4-(5-dibenzo(a,e)cycloheptatrienylidene)piperidine; 1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)piperidine; 4-(5-Dibenzo(a,d)cyclohepten-5-ylidine)-1-methylpiperidine; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidene; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidine; 4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine; 4-(5H-dibenzo[a,d][7]annulen-5-ylidene)-1-methylpiperidine; 5-(1-Methylpiperidylidene-4)-5H-dibenzo(a,d)cyclopheptene
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Rhinitis [ICD-11: FA20; ICD-10: H10] | Approved | [1] | |
| Therapeutic Class |
Antihistamines
|
|||
| Company |
Merck & Co
|
|||
| Structure |
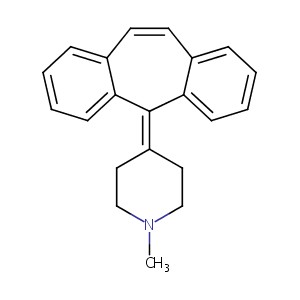 |
Download2D MOL |
||
| Formula |
C21H21N
|
|||
| Canonical SMILES |
CN1CCC(=C2C3=CC=CC=C3C=CC4=CC=CC=C42)CC1
|
|||
| InChI |
1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3
|
|||
| InChIKey |
JJCFRYNCJDLXIK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 129-03-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9151, 597387, 3206368, 4509244, 7979013, 8151866, 10506298, 11110964, 11110965, 11113362, 11466131, 11467251, 11485761, 14751189, 26751603, 29222066, 46508613, 47216945, 47440433, 47440434, 47515490, 47589155, 47736665, 48110626, 48110627, 48185155, 48259431, 48415832, 49698345, 50015917, 50100203, 50104260, 51092057, 78634349, 85209714, 85279540, 85787813, 85788782, 90341656, 92309316, 92729717, 93166482, 94564370, 96079554, 103173872, 103928989, 104171133, 104301985, 108667051, 116933607
|
|||
| ChEBI ID |
CHEBI:4046
|
|||
| ADReCS Drug ID | BADD_D00552 ; BADD_D00553 | |||
| SuperDrug ATC ID |
R06AX02
|
|||
| SuperDrug CAS ID |
cas=000129033
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides uniformis
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides uniformis was decreased by Cyproheptadine hydrochloride (adjusted p-values: 1.18E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [3], [4] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 3 | Cyproheptadine displays preclinical activity in myeloma and leukemia. Blood. 2008 Aug 1;112(3):760-9. | |||
| REF 4 | Antihistamines in the treatment of dermatitis. J Cutan Med Surg. 2003 Nov-Dec;7(6):467-73. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

