Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00YPR
|
|||
| Former ID |
DCL000349
|
|||
| Drug Name |
CP-547632
|
|||
| Synonyms |
BFF; CP 547632; CP-547,632; 3-(4-Bromo-2,6-difluorobenzyloxy)-5-(3-(4-pyrrolidin-1-ylbutyl)ureido)isothiazole-4-carboxylic acid amide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Non-small-cell lung cancer [ICD-11: 2C25.Y] | Phase 2 | [1], [2] | |
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56] | Phase 2 | [1], [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Phase 2 | [1], [2] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
OSI Pharmaceuticals
|
|||
| Structure |
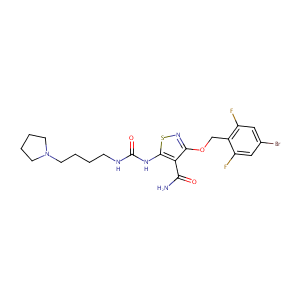 |
Download2D MOL |
||
| Formula |
C20H24BrF2N5O3S
|
|||
| Canonical SMILES |
C1CCN(C1)CCCCNC(=O)NC2=C(C(=NS2)OCC3=C(C=C(C=C3F)Br)F)C(=O)N
|
|||
| InChI |
1S/C20H24BrF2N5O3S/c21-12-9-14(22)13(15(23)10-12)11-31-18-16(17(24)29)19(32-27-18)26-20(30)25-5-1-2-6-28-7-3-4-8-28/h9-10H,1-8,11H2,(H2,24,29)(H2,25,26,30)
|
|||
| InChIKey |
HXHAJRMTJXHJJZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 252003-65-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14787667, 24117408, 44847595, 56374257, 75180365, 89449533, 99032984, 103561898, 103905622, 125569056, 126665919, 128126388, 134339001, 135253187, 136946546, 137242797, 137275944, 143360656, 144115846, 152343781, 160838432, 162011598, 163098917, 163403702, 163847057, 164045828, 174528562, 180190847, 198984058, 204406860, 223366210, 223395101, 223665940, 223740279, 226547130, 242060307, 247504149, 249814520, 249860726, 250213353, 251963041, 252070376, 252160342, 252215194, 252435623, 252552606
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7881). | |||
| REF 2 | ClinicalTrials.gov (NCT00096239) CP-547,632 in Treating Patients With Recurrent or Persistent Ovarian Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer. U.S. National Institutes of Health. | |||
| REF 3 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
| REF 4 | YM-359445, an orally bioavailable vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor, has highly potent antitumor activity against established tumors. Clin Cancer Res. 2006 Mar 1;12(5):1630-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

