Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01BYB
|
|||
| Former ID |
DAP000474
|
|||
| Drug Name |
Adenosine triphosphate
|
|||
| Synonyms |
Adenylpyrophosphate; Triphosadenine; Triphosadenine (DCF); Adenosine 5'-triphosphate(4-); [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate; [[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl]oxy-oxidophosphoryl] phosphate; [[[(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxyphosphonic acid; 3b2q; 5-(6-Aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-ylmethoxy-hydroxy-phosphoryloxy-hydroxy-phosphoryl oxyphosphonic acid; 9H-purin-6-amine, 9-[5-O-[hydroxy[[hydroxy(phosphonooxy)phosphinyl]oxy]phosphinyl]
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Malnutrition [ICD-11: 5B50-5B71] | Approved | [1] | |
| Heart disease [ICD-11: BA41-BA42; ICD-9: 390-429] | Phase 2 | [2] | ||
| Bradycardia [ICD-11: MC81.1; ICD-10: R00.1] | Discontinued in Phase 2 | [3] | ||
| Therapeutic Class |
Dietary supplement
|
|||
| Structure |
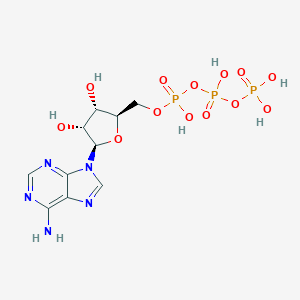 |
Download2D MOL |
||
| Formula |
C10H16N5O13P3
|
|||
| Canonical SMILES |
C1=NC(=C2C(=N1)N(C=N2)C3C(C(C(O3)COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O)O)N
|
|||
| InChI |
1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1
|
|||
| InChIKey |
ZKHQWZAMYRWXGA-KQYNXXCUSA-N
|
|||
| CAS Number |
CAS 56-65-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
2157, 8148096, 11688362, 24877532, 39443564, 49737792, 50247497, 53812597, 53812679, 53812681, 53812739, 53812902, 53812904, 56412694, 56436522, 60849405, 84970651, 85145927, 87557452, 87557505, 87557712, 104075349, 114001216, 131319136, 137251856, 164151282, 170481218, 170481265, 170481338, 170481406, 170481407, 170481627, 170481825, 170482177, 170482179, 170482197, 170482263, 170483168, 170483402, 170483906, 170483907, 170484256, 170485046, 170485047, 170485048, 223501249
|
|||
| ChEBI ID |
CHEBI:15422
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Emerging drugs for chemotherapy-induced mucositis. Expert Opin Emerg Drugs. 2008 Sep;13(3):511-22. | |||
| REF 2 | Duska Therapeutics Updates Recent Drug Development and Corporate Activities. U.S. Securities and Exchange Commission. February 16, 2005. | |||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023552) | |||
| REF 4 | Targeted chronic myeloid leukemia therapy: Seeking a cure. Am J Health Syst Pharm. 2007 Dec 15;64(24 Suppl 15):S9-15. | |||
| REF 5 | High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002 May 1;99(9):3472-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

