Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01TMQ
|
|||
| Former ID |
DCL000733
|
|||
| Drug Name |
Bupropion+naltrexone
|
|||
| Synonyms |
Mysimba; Contrave; Naltrexone/bupropion; Naltrexone / bupropion; Bupropion / naltrexone; Bupropion mixture with naltrexone; SCHEMBL15633271; 1201668-08-7
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Obesity [ICD-11: 5B81; ICD-10: E66; ICD-9: 278] | Phase 3 | [1] | |
| Company |
Orexigen Therapeutics
|
|||
| Structure |
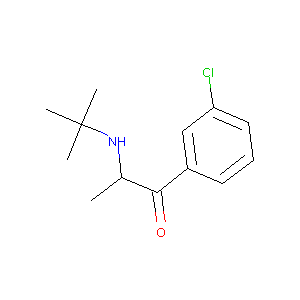 |
Download2D MOL |
||
| Formula |
C33H41ClN2O5
|
|||
| Canonical SMILES |
CC(C(=O)C1=CC(=CC=C1)Cl)NC(C)(C)C.C1CC1CN2CCC34C5C(=O)CCC3(C2CC6=C4C(=C(C=C6)O)O5)O
|
|||
| InChI |
1S/C20H23NO4.C13H18ClNO/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11;1-9(15-13(2,3)4)12(16)10-6-5-7-11(14)8-10/h3-4,11,15,18,22,24H,1-2,5-10H2;5-9,15H,1-4H3/t15-,18+,19+,20-;/m1./s1
|
|||
| InChIKey |
KVNBDVQGENTICK-ITLPAZOVSA-N
|
|||
| CAS Number |
CAS 1201668-08-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Dopamine transporter (DAT) | Target Info | Inhibitor | [2] |
| Norepinephrine transporter (NET) | Target Info | Inhibitor | [2] | |
| Opioid receptor (OPR) | Target Info | Antagonist | [2] | |
| KEGG Pathway | Dopaminergic synapse | |||
| Parkinson's disease | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| Parkinson disease | ||||
| Dopamine receptor mediated signaling pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | |||
| Reactome | Na+/Cl- dependent neurotransmitter transporters | |||
| WikiPathways | Monoamine Transport | |||
| NRF2 pathway | ||||
| Dopaminergic Neurogenesis | ||||
| Parkinsons Disease Pathway | ||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | ||||
| Neurotransmitter Clearance In The Synaptic Cleft | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013 May;21(5):935-43. | |||
| REF 2 | Anti-obesity drugs. Expert Opin Pharmacother. 2008 Jun;9(8):1339-50. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

