Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02HBB
|
|||
| Former ID |
DNCL002590
|
|||
| Drug Name |
AEZS-108
|
|||
| Synonyms |
Zoptarelin doxorubicin; AEZS-108; AN-152; UNII-27844X2J29; ZEN-008; 27844X2J29; Zoptrex; Zoptarelin doxorubicin [INN]; Zoptarelin doxorubicin [USAN]; AEZS 108; CHEMBL3544954; AN 152; Luteinizing hormone-releasing factor (pig), 6-(N6-(5-(2-(4-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl)-2-oxoethoxy)-1,5-dioxopentyl)-D-lysine)-, (2S-cis)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute lymphoblastic leukaemia [ICD-11: 2A85; ICD-10: C85.1, C88.7] | Phase 3 | [1] | |
| Company |
AEterna Zentaris
|
|||
| Structure |
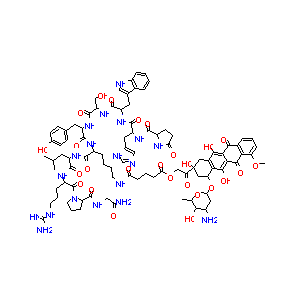 |
Download2D MOL |
||
| Formula |
C91H117N19O26
|
|||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)COC(=O)CCCC(=O)NCCCCC(C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)N6CCCC6C(=O)NCC(=O)N)NC(=O)C(CC7=CC=C(C=C7)O)NC(=O)C(CO)NC(=O)C(CC8=CNC9=CC=CC=C98)NC(=O)C(CC1=CN=CN1)NC(=O)C1CCC(=O)N1)O)N)O
|
|||
| InChI |
1S/C91H117N19O26/c1-44(2)31-58(83(125)104-57(17-11-29-98-90(94)95)89(131)110-30-12-18-63(110)88(130)100-40-67(93)114)105-81(123)55(16-7-8-28-97-68(115)20-10-21-70(117)134-42-66(113)91(132)36-52-73(65(37-91)136-71-35-53(92)76(118)45(3)135-71)80(122)75-74(78(52)120)77(119)51-14-9-19-64(133-4)72(51)79(75)121)103-84(126)59(32-46-22-24-49(112)25-23-46)106-87(129)62(41-111)109-85(127)60(33-47-38-99-54-15-6-5-13-50(47)54)107-86(128)61(34-48-39-96-43-101-48)108-82(124)56-26-27-69(116)102-56/h5-6,9,13-15,19,22-25,38-39,43-45,53,55-63,65,71,76,99,111-112,118,120,122,132H,7-8,10-12,16-18,20-21,26-37,40-42,92H2,1-4H3,(H2,93,114)(H,96,101)(H,97,115)(H,100,130)(H,102,116)(H,103,126)(H,104,125)(H,105,123)(H,106,129)(H,107,128)(H,108,124)(H,109,127)(H4,94,95,98)/t45-,53-,55+,56-,57-,58-,59-,60-,61-,62-,63-,65-,71-,76+,91-/m0/s1
|
|||
| InChIKey |
OOUACICUAVTCEC-LZHWUUGESA-N
|
|||
| CAS Number |
CAS 139570-93-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Gonadotropin-releasing hormone receptor (GNRHR) | Target Info | Modulator | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| GnRH signaling pathway | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| IL2 Signaling Pathway | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||
| Reactome | Hormone ligand-binding receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Gastrin-CREB signalling pathway via PKC and MAPK | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01767155) Study Comparing AEZS-108/ZoptEC (Zoptarelin Doxorubicin in Endometrial Cancer) to Doxorubicin as a Second Line Therapy of Endometrial Cancer. U.S. National Institutesof Health. | |||
| REF 2 | Stability of cytotoxic luteinizing hormone-releasing hormone conjugate (AN-152) containing doxorubicin 14-O-hemiglutarate in mouse and human serum in vitro: implications for the design of preclinicalstudies. Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):829-34. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

