Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02NAW
|
|||
| Former ID |
DNC010913
|
|||
| Drug Name |
N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3',4'-DIHYDROSPIRO[CYCLOHEXANE-1,2'-PYRANO[2,3-B]PYRIDINE]-4'-YL)-2-HYDROXY-2-METHYLPROPANAMIDE (ENANTIOMERIC MIX)
|
|||
| Synonyms |
CHEMBL1085566; SCHEMBL1404756; BDBM50320185; N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3',4'-DIHYDROSPIRO[CYCLOHEXANE-1,2'-PYRANO[2,3-B]PYRIDINE]-4'-YL)-2-HYDROXY-2-METHYLPROPANAMIDE (ENANTIOMERIC MIX); N-(7''-(2-CHLOROPHENYL)-6''-(4-CHLOROPHENYL)-3'',4''-DIHYDROSPIRO[CYCLOHEXANE-1,2''-PYRANO[2,3-B]PYRIDINE]-4''-YL)-2-HYDROXY-2-METHYLPROPANAMIDE (ENANTIOMERIC MIX)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
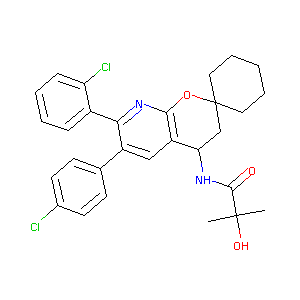 |
Download2D MOL |
||
| Formula |
C29H30Cl2N2O3
|
|||
| Canonical SMILES |
CC(C)(C(=O)NC1CC2(CCCCC2)OC3=C1C=C(C(=N3)C4=CC=CC=C4Cl)C5=CC=C(C=C5)Cl)O
|
|||
| InChI |
1S/C29H30Cl2N2O3/c1-28(2,35)27(34)32-24-17-29(14-6-3-7-15-29)36-26-22(24)16-21(18-10-12-19(30)13-11-18)25(33-26)20-8-4-5-9-23(20)31/h4-5,8-13,16,24,35H,3,6-7,14-15,17H2,1-2H3,(H,32,34)
|
|||
| InChIKey |
UTPSWDIIQDVVTK-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cannabinoid receptor 1 (CB1) | Target Info | Inhibitor | [1] |
| Cannabinoid receptor 2 (CB2) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Rap1 signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | ||||
| Panther Pathway | Endogenous cannabinoid signaling | |||
| Pathway Interaction Database | N-cadherin signaling events | |||
| Reactome | Class A/1 (Rhodopsin-like receptors) | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Small Ligand GPCRs | ||||
| BDNF signaling pathway | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Dihydro-pyrano[2,3-b]pyridines and tetrahydro-1,8-naphthyridines as CB1 receptor inverse agonists: synthesis, SAR and biological evaluation. Bioorg Med Chem Lett. 2010 Jun 15;20(12):3750-4. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

