Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02RDX
|
|||
| Former ID |
DIB014515
|
|||
| Drug Name |
XP-23829
|
|||
| Synonyms |
Methylhydrogenfumarate prodrug (multiple sclerosis), XenoPort
Click to Show/Hide
|
|||
| Indication | Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Phase 2 | [1] | |
| Plaque psoriasis [ICD-11: EA90.0; ICD-10: L40.0] | Phase 2 | [2] | ||
| Company |
Xenoport
|
|||
| Structure |
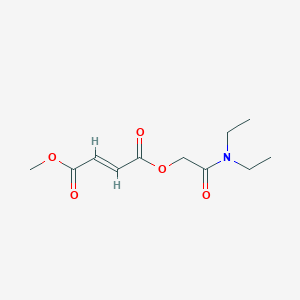 |
Download2D MOL |
||
| Formula |
C11H17NO5
|
|||
| Canonical SMILES |
CCN(CC)C(=O)COC(=O)C=CC(=O)OC
|
|||
| InChI |
1S/C11H17NO5/c1-4-12(5-2)9(13)8-17-11(15)7-6-10(14)16-3/h6-7H,4-5,8H2,1-3H3/b7-6+
|
|||
| InChIKey |
AKUGRXRLHCCENI-VOTSOKGWSA-N
|
|||
| CAS Number |
CAS 1208229-58-6
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02173301) A Study to Assess the Efficacy and Safety of XP23829 in Subjects With Moderate-to-Severe Chronic Plaque-Type Psoriasis. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036491) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

