Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02YKI
|
|||
| Former ID |
DIB002902
|
|||
| Drug Name |
PF-04995274
|
|||
| Synonyms |
UNII-XI179PG9LV; PF 04995274; XI179PG9LV; 1331782-27-4; CHEMBL2152922; (R)-4-((4-(((4-(Tetrahydrofuran-3-yloxy)-1,2-benzisoxazol-3-yl)oxy)methyl)piperidin-1-yl)methyl)tetrahydro-2H-pyran-4-ol; PF04995274; compound 2d [PMID: 22974325]; SCHEMBL619629; GTPL9059; WLLOFQROROXOMO-GOSISDBHSA-N; ZINC95577747; BDBM50398598; DB12675; NCGC00386746-01; PF-04995274, > 4-[[4-[[4-[(3R)-oxolan-3-yl]oxy-1,2-benzoxazol-3-yl]oxymethyl]piperidin-1-yl]methyl]oxan-4-ol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 1 | [1] | |
| Company |
Pfizer Inc
|
|||
| Structure |
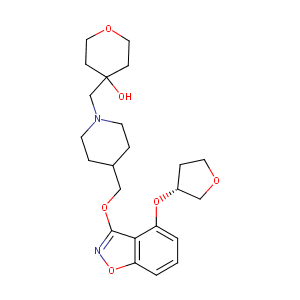 |
Download2D MOL |
||
| Formula |
C23H32N2O6
|
|||
| Canonical SMILES |
C1CN(CCC1COC2=NOC3=C2C(=CC=C3)OC4CCOC4)CC5(CCOCC5)O
|
|||
| InChI |
1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1
|
|||
| InChIKey |
WLLOFQROROXOMO-GOSISDBHSA-N
|
|||
| CAS Number |
CAS 1331782-27-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 4 receptor (HTR4) | Target Info | Agonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT4 type receptor mediated signaling pathway | ||||
| Pathwhiz Pathway | Excitatory Neural Signalling Through 5-HTR 4 and Serotonin | |||
| Reactome | Serotonin receptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Serotonin Receptor 4/6/7 and NR3C Signaling | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01193062) Study In Healthy Subjects To Evaluate The Changes In The Protein sAPP-Alpha In Cerebrospinal Fluid Following A Single Oral Dose Of PF-04995274. U.S. National Institutes of Health. | |||
| REF 2 | Pharmacokinetics, safety and tolerability of PF04995274: A 5HT4 partial agonist being developed for the treatment of Alzheimer's disease. Alzheimer's and Dementia. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

