Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03FNJ
|
|||
| Former ID |
DAP000988
|
|||
| Drug Name |
Pipobroman
|
|||
| Synonyms |
Amedel; Pipobromanum; Vercyte; A 1803; A-8103; Pipobroman [USAN:INN]; Pipobromanum [INN-Latin]; VERCYTE (TN); Pipobroman (JAN/USAN/INN); N,N'-Bis(3-bromopropionyl)piperazine; N,N-Bis-(3-bromopropionyl)-piperazine; 1,4-Bis(3-bromopropanoyl)piperazine; 1,4-Bis(3-bromopropionyl)piperazine; 3-bromo-1-[4-(3-bromopropanoyl)piperazin-1-yl]propan-1-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Refractory chronic myeloid leukaemia [ICD-11: 2A20; ICD-10: D47.7; ICD-9: 205.1] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
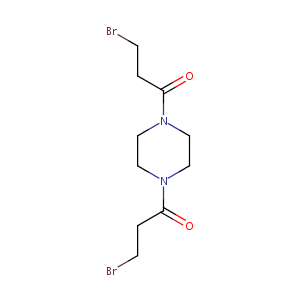 |
Download2D MOL |
||
| Formula |
C10H16Br2N2O2
|
|||
| Canonical SMILES |
C1CN(CCN1C(=O)CCBr)C(=O)CCBr
|
|||
| InChI |
1S/C10H16Br2N2O2/c11-3-1-9(15)13-5-7-14(8-6-13)10(16)2-4-12/h1-8H2
|
|||
| InChIKey |
NJBFOOCLYDNZJN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 54-91-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9566, 86412, 220314, 5518519, 7847533, 8149987, 8152976, 10526660, 11335974, 11361213, 11364621, 11367183, 11369745, 11371894, 11377907, 11406401, 11462185, 11484520, 11488728, 11490862, 11495541, 14876527, 26612478, 26680378, 26748793, 26748794, 26748795, 29223923, 46506548, 47216809, 47515347, 47589037, 48416444, 50011390, 50107466, 50733206, 57322482, 92124614, 92307921, 99301887, 103589881, 104307586, 121365257, 124637578, 128709591, 134222726, 134338258, 134971949, 137006686, 137545237
|
|||
| ChEBI ID |
CHEBI:8242
|
|||
| SuperDrug ATC ID |
L01AX02
|
|||
| SuperDrug CAS ID |
cas=000054911
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Binder | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7271). | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 016245. | |||
| REF 4 | Effects of piposulfan (Ancyte) on protein and DNA synthesis in Ehrlich ascites carcinoma. Life Sci II. 1971 Jun 8;10(11):605-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

