Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03GAX
|
|||
| Former ID |
DIB000037
|
|||
| Drug Name |
TAK-802
|
|||
| Indication | Urinary dysfunction [ICD-11: GC2Z; ICD-10: N39.3-N39.4] | Discontinued in Phase 2 | [1] | |
| Company |
Takeda Pharmaceutical Co Ltd
|
|||
| Structure |
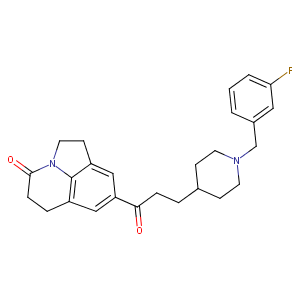 |
Download2D MOL |
||
| Formula |
C26H29FN2O2
|
|||
| Canonical SMILES |
C1CN(CCC1CCC(=O)C2=CC3=C4C(=C2)CCN4C(=O)CC3)CC5=CC(=CC=C5)F
|
|||
| InChI |
1S/C26H29FN2O2/c27-23-3-1-2-19(14-23)17-28-11-8-18(9-12-28)4-6-24(30)22-15-20-5-7-25(31)29-13-10-21(16-22)26(20)29/h1-3,14-16,18H,4-13,17H2
|
|||
| InChIKey |
RAYMNBAAUXRZHA-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Acetylcholinesterase (AChE) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Cholinergic synapse | ||||
| Panther Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | ||||
| Pathwhiz Pathway | Phospholipid Biosynthesis | |||
| Pathway Interaction Database | ATF-2 transcription factor network | |||
| WikiPathways | Monoamine Transport | |||
| Biogenic Amine Synthesis | ||||
| Acetylcholine Synthesis | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020145) | |||
| REF 2 | Effects of TAK-802, a novel acetylcholinesterase inhibitor, on distension-induced rhythmic bladder contractions in rats and guinea pigs. Eur J Pharmacol. 2004 Feb 6;485(1-3):299-305. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

