Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03HYQ
|
|||
| Former ID |
DAP000875
|
|||
| Drug Name |
Ranolazine
|
|||
| Synonyms |
RANOLAZINE; 95635-55-5; Ranexa; N-(2,6-dimethylphenyl)-2-(4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)piperazin-1-yl)acetamide; CVT-303; (-)-Ranolazine; Ranolazine (Ranexa); Ranolazine 2HCl; RS-43285-003; 142387-99-3; N-(2,6-dimethylphenyl)-2-[4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl]acetamide; CHEBI:87690; N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl}acetamide; C24H33N3O4; NCGC00015897-02; DSSTox_RID_80743; DSSTox_CID_25196; DSSTox_GSID_45196; Ran4; CVT 303; Latixa; RAN D; Latixa; Ranolazine dihydrochloride; KEG-1295; RS-43285; Ranexa (TN); Ranexa, Ranolazine; Ranolazine (USAN/INN); Ranolazine extended-release; Ranolazine/Dronedarone Fixed-Dose Combination
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic/stable angina [ICD-11: BA40.1; ICD-9: 413] | Approved | [1], [2], [3] | |
| Paroxysmal atrial fibrillation [ICD-11: BC81.30; ICD-10: I48.0; ICD-9: 427.31] | Phase 2 | [4] | ||
| Therapeutic Class |
Analgesics
|
|||
| Company |
CV Therapeutics
|
|||
| Structure |
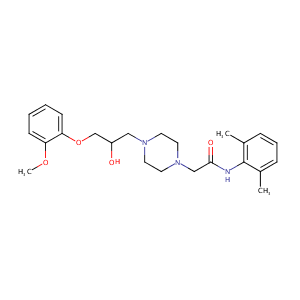 |
Download2D MOL |
||
| Formula |
C24H33N3O4
|
|||
| Canonical SMILES |
CC1=C(C(=CC=C1)C)NC(=O)CN2CCN(CC2)CC(COC3=CC=CC=C3OC)O
|
|||
| InChI |
1S/C24H33N3O4/c1-18-7-6-8-19(2)24(18)25-23(29)16-27-13-11-26(12-14-27)15-20(28)17-31-22-10-5-4-9-21(22)30-3/h4-10,20,28H,11-17H2,1-3H3,(H,25,29)
|
|||
| InChIKey |
XKLMZUWKNUAPSZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 95635-55-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7980486, 8184974, 12013498, 14856376, 26612929, 26749060, 26749061, 43114706, 46505145, 47207361, 47795040, 48169373, 50070803, 50107515, 51004683, 56463697, 57313861, 81040983, 85171832, 85789241, 90340842, 92124680, 92307911, 92309225, 92712119, 103482101, 104171232, 104253386, 104311416, 117573069, 121363701, 124750209, 124757504, 124799345, 124881338, 124881339, 125164308, 125360240, 126583887, 126622101, 126630205, 126656915, 126666839, 126724363, 129701807, 134338152, 135015431, 135684171, 135692283, 136375567
|
|||
| ChEBI ID |
CHEBI:87690
|
|||
| ADReCS Drug ID | BADD_D01915 | |||
| SuperDrug ATC ID |
C01EB18
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7291). | |||
| REF 2 | 2006 drug approvals: finding the niche. Nat Rev Drug Discov. 2007 Feb;6(2):99-101. | |||
| REF 3 | Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006 Jul;92 Suppl 4:iv1-iv5. | |||
| REF 4 | Clinical pipeline report, company report or official report of Gilead. | |||
| REF 5 | Tranexamic acid in trauma: how should we use it. J Trauma Acute Care Surg. 2013 Jun;74(6):1575-86. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

