Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03JTG
|
|||
| Former ID |
DIB010509
|
|||
| Drug Name |
Fruquintinib
|
|||
| Synonyms |
HMPL-013
Click to Show/Hide
|
|||
| Indication | Colorectal cancer [ICD-11: 2B91.Z] | Approved | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 3 | [2] | ||
| Company |
Takeda
|
|||
| Structure |
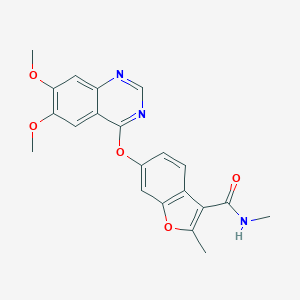 |
Download2D MOL |
||
| Formula |
C21H19N3O5
|
|||
| Canonical SMILES |
CC1=C(C2=C(O1)C=C(C=C2)OC3=NC=NC4=CC(=C(C=C43)OC)OC)C(=O)NC
|
|||
| InChI |
1S/C21H19N3O5/c1-11-19(20(25)22-2)13-6-5-12(7-16(13)28-11)29-21-14-8-17(26-3)18(27-4)9-15(14)23-10-24-21/h5-10H,1-4H3,(H,22,25)
|
|||
| InChIKey |
BALLNEJQLSTPIO-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1194506-26-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Vascular endothelial growth factor receptor (VEGFR) | Target Info | Inhibitor | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 217564 | |||
| REF 2 | ClinicalTrials.gov (NCT02314819) A Phase III Trial Evaluating Fruquintinib Efficacy and Safety in 3+ Line Colorectal Cancer Patients RESCO). U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

