Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04BQR
|
|||
| Former ID |
DIB003267
|
|||
| Drug Name |
Acecainide
|
|||
| Synonyms |
N-Acetylprocainamide; Acekainid; 32795-44-1; Acecainida; Acecainidum; NAPA; N-Acetyloprokainamid; Acecainide [INN]; Acekainid [Polish]; 4'-((2-(Diethylamino)ethyl)carbamoyl)acetanilide; Acecainidum [INN-Latin]; Acecainida [INN-Spanish]; N-Acetyloprokainamid [Polish]; UNII-910Q707V6F; CHEBI:60728; Benzamide, 4-(acetylamino)-N-(2-(diethylamino)ethyl)-; BRN 2868559; CHEMBL1097; MLS000069490; KEECCEWTUVWFCV-UHFFFAOYSA-N; 4-acetamido-N-[2-(diethylamino)ethyl]benzamide; 910Q707V6F; SMR000059070; ACETANILIDE,; NAPA; Acecainide hydrochloride; ASL-601; Acetylprocainamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Discontinued in Phase 3 | [1] | |
| Company |
King Pharmaceuticals R&D Inc
|
|||
| Structure |
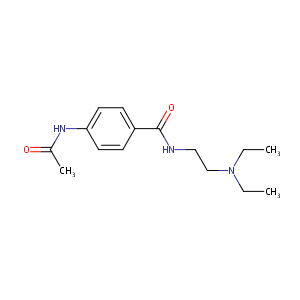 |
Download2D MOL |
||
| Formula |
C15H23N3O2
|
|||
| Canonical SMILES |
CCN(CC)CCNC(=O)C1=CC=C(C=C1)NC(=O)C
|
|||
| InChI |
1S/C15H23N3O2/c1-4-18(5-2)11-10-16-15(20)13-6-8-14(9-7-13)17-12(3)19/h6-9H,4-5,10-11H2,1-3H3,(H,16,20)(H,17,19)
|
|||
| InChIKey |
KEECCEWTUVWFCV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 32795-44-1
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:60728
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides fragilis ATCC43859
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Acecainide can be metabolized by Bacteroides fragilis ATCC43859 (log2FC = -0.345; p = 0.031). | |||
|
Studied Microbe: Bacteroides uniformis ATCC 8492
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Acecainide can be metabolized by Bacteroides uniformis ATCC 8492 (log2FC = -0.376; p = 0.009). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Potassium channel unspecific (KC) | Target Info | Opener | [3] |
| Voltage-gated sodium channel alpha Nav1.5 (SCN5A) | Target Info | Blocker | [4] | |
| KEGG Pathway | Adrenergic signaling in cardiomyocytes | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Cardiac Progenitor Differentiation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000315) | |||
| REF 2 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||
| REF 3 | Acecainide (N-acetylprocainamide). A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cardiac arrhythmias. Drugs. 1990 May;39(5):720-40. | |||
| REF 4 | Monoamine transporter and sodium channel mechanisms in the rapid pressor response to cocaine. Pharmacol Biochem Behav. 1998 Feb;59(2):305-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

