Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04HQK
|
|||
| Former ID |
DNC012155
|
|||
| Drug Name |
N-(5-Sulfamoyl-[1,3,4]thiadiazol-2-yl)-benzamide
|
|||
| Synonyms |
NSC698990; CHEMBL281376; N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)benzamide; 86029-44-9; N-(2-sulfamoyl-1,3,4-thiadiazol-5-yl)benzamide; Benzamide, N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-; SCHEMBL9795960; AC1L997Y; BDBM16669; CTK2I3789; DTXSID30327878; ZINC5925092; heterocyclic sulfonamide compound 34; AKOS030579978; NSC-698990; NCI60_035393; LS-25666; 2-benzoylamino-1,3,4-thiadiazole 5-sulfonamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
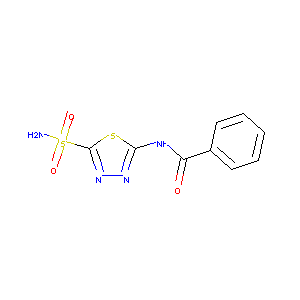 |
Download2D MOL |
||
| Formula |
C9H8N4O3S2
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C(=O)NC2=NN=C(S2)S(=O)(=O)N
|
|||
| InChI |
1S/C9H8N4O3S2/c10-18(15,16)9-13-12-8(17-9)11-7(14)6-4-2-1-3-5-6/h1-5H,(H2,10,15,16)(H,11,12,14)
|
|||
| InChIKey |
PMNNJWNEWDGYIV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 86029-44-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Carbonic anhydrase IV (CA-IV) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Nitrogen metabolism | |||
| Proximal tubule bicarbonate reclamation | ||||
| Reactome | Erythrocytes take up carbon dioxide and release oxygen | |||
| Erythrocytes take up oxygen and release carbon dioxide | ||||
| Reversible hydration of carbon dioxide | ||||
| WikiPathways | Reversible Hydration of Carbon Dioxide | |||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | ||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valproyl and other lipophilic moieties. J Med Chem. 2002 Jan 17;45(2):312-20. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

