Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04QLR
|
|||
| Former ID |
DAP000509
|
|||
| Drug Name |
Dyclonine
|
|||
| Synonyms |
Diclonia; Diclonina; Dyclocaine; Dyclocainum; Dyclonin; Dycloninum; Dyclothane; Tanaclone; Diclonina [INN-Spanish]; Dyclonine (INN); Dyclonine [INN:BAN]; Dycloninum [INN-Latin]; 1-(4-Butoxy-phenyl)-3-piperidin-1-yl-propan-1-one; 1-(4-Butoxyphenyl)-3-(1-piperidinyl)-1-propanone; 1-(4-butoxyphenyl)-3-(piperidin-1-yl)propan-1-one; 1-(4-butoxyphenyl)-3-piperidin-1-ylpropan-1-one; 1-Propanone, 1-(4-butoxyphenyl)-3-(1-piperidinyl)-(9CI); 2-(1-piperidyl)ethyl p-butoxyphenyl ketone; 3-Piperidino-4'-butoxypropiophenone; 4'-Butoxy-3-piperidinopropiophenone; 4-butoxy-beta-piperidinopropiophenone; 4-n-butoxy-beta-(1-piperidyl)propiophenone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Pain [ICD-11: MG30-MG3Z] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Anesthetics
|
|||
| Company |
Astrazeneca Lp
|
|||
| Structure |
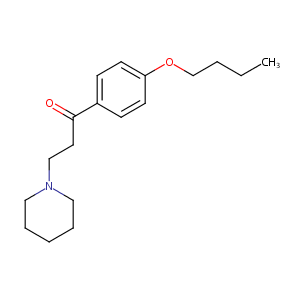 |
Download2D MOL |
||
| Formula |
C18H27NO2
|
|||
| Canonical SMILES |
CCCCOC1=CC=C(C=C1)C(=O)CCN2CCCCC2
|
|||
| InChI |
1S/C18H27NO2/c1-2-3-15-21-17-9-7-16(8-10-17)18(20)11-14-19-12-5-4-6-13-19/h7-10H,2-6,11-15H2,1H3
|
|||
| InChIKey |
BZEWSEKUUPWQDQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 586-60-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10083, 7726237, 7979140, 8152025, 10589257, 11112403, 11335807, 11361046, 11363910, 11366472, 11369034, 11371260, 11374276, 11377196, 11462018, 11466292, 11467412, 11484901, 11486063, 11488829, 11490226, 11492344, 11494830, 15270786, 25667259, 29222322, 46506697, 47216771, 47515313, 47736472, 47885405, 48035111, 48035112, 48110446, 49698861, 50026050, 50100427, 50713692, 57321651, 85789355, 92711541, 96024577, 99319105, 104302759, 105052237, 117720385, 123090168, 124596734, 124882339, 124882340
|
|||
| ChEBI ID |
CHEBI:4724
|
|||
| SuperDrug ATC ID |
N01BX02; R02AD04
|
|||
| SuperDrug CAS ID |
cas=000586607
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated sodium channel alpha Nav1.5 (SCN5A) | Target Info | Blocker | [4] |
| KEGG Pathway | Adrenergic signaling in cardiomyocytes | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Cardiac Progenitor Differentiation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7173). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 009925. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Monoamine transporter and sodium channel mechanisms in the rapid pressor response to cocaine. Pharmacol Biochem Behav. 1998 Feb;59(2):305-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

