Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04SIU
|
|||
| Former ID |
DIB004144
|
|||
| Drug Name |
MCD-386
|
|||
| Synonyms |
CDD-0102; CDD-0262; CDD-0264; CDD-102; MCD-386 Forte; MCD-386 Transderm; MCD-386CR; MCD-386 Forte/Transderm; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), Cognitive Pharmaceuticals; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), University of Toledo; Tetrahydropyrimidine muscarinicM1 agonists (Alzheimer's disease), Mithridion; MCD-386 (oral controlled release, CNS disorders), Mithridion; MCD-386 (high dose transdermal, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (high dose, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (transdermal, Alzheimer's disease/schizophrenia), Mithridion
Click to Show/Hide
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 1 | [1] | |
| Company |
Mithridion Inc; University of Toledo
|
|||
| Structure |
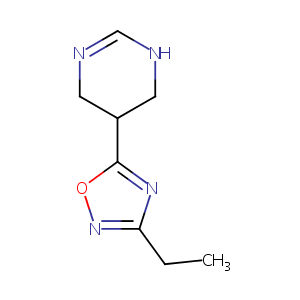 |
Download2D MOL |
||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009639) | |||
| REF 2 | The processing of the selective M1 agonist CDD-0102-J by human hepatic drug metabolizing enzymes. Am J Ther. 2005 Jul-Aug;12(4):300-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

