Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04ZKY
|
|||
| Former ID |
DIB003124
|
|||
| Drug Name |
SEP-363856
|
|||
| Indication | Schizophrenia [ICD-11: 6A20] | Phase 3 | [1] | |
| Parkinson disease [ICD-11: 8A00.0; ICD-9: 332] | Phase 2 | [2], [3] | ||
| Major depressive disorder [ICD-11: 6A70.3; ICD-10: F32.2] | Phase 1 | [4] | ||
| Company |
Sunovion
|
|||
| Structure |
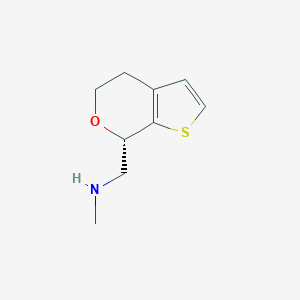 |
Download2D MOL |
||
| Formula |
C9H13NOS
|
|||
| Canonical SMILES |
CNCC1C2=C(CCO1)C=CS2
|
|||
| InChI |
1S/C9H13NOS/c1-10-6-8-9-7(2-4-11-8)3-5-12-9/h3,5,8,10H,2,4,6H2,1H3/t8-/m0/s1
|
|||
| InChIKey |
ABDDQTDRAHXHOC-QMMMGPOBSA-N
|
|||
| CAS Number |
CAS 1310426-33-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 1A receptor (HTR1A) | Target Info | Agonist | [2] |
| Trace amine-associated receptor-1 (TAAR1) | Target Info | Agonist | [2] | |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT1 type receptor mediated signaling pathway | ||||
| Reactome | Serotonin receptors | |||
| G alpha (i) signalling events | ||||
| G alpha (s) signalling events | ||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | |||
| SIDS Susceptibility Pathways | ||||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04109950) A Clinical Study to Evaluate the Long-term Safety and Tolerability of an Investigational Drug in People With Schizophrenia. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | ClinicalTrials.gov (NCT01994473) Study Assessing the Safety, Tolerability, and Pharmacokinetics of SEP-363856 in Male and Female Subjects With Schizophrenia. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

