Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05EJG
|
|||
| Former ID |
DAP001316
|
|||
| Drug Name |
L-Tryptophan
|
|||
| Synonyms |
Ardeytropin; Kalma; LTR; Lyphan; Optimax; Pacitron; Sedanoct; Triptofano; Trofan; Trp; Tryptacin; Tryptan; Tryptophane; Tryptophanum; Triptofano [Spanish]; Tryptophane [French]; Tryptophanum [Latin]; EH 121; MT1; T 0254; Alti-Tryptophan; L-TRYPTOPHAN SIGMA GRADE; L-Trp; L-Tryptofan; L-Tryptophane; L-Ttp; TRP NH3+ COOH; TRP-01; Tryptophan (VAN); Tryptophan [USAN:INN]; H-Trp-oh; Indole-3-alanine; L-Tryptophan (9CI); L-Tryptophan (JP15); Tryptophan (H-3); Tryptophan (USP/INN); L-b-3-Indolylalanine; L-beta-3-Indolylalanine; Tryptophan, L-(8CI); Alanine, 3-indol-3-yl; Alpha'-Amino-3-indolepropionic acid; L-(-)-Tryptophan; L-(-)-Tryptophane; L-a-Aminoindole-3-propionic acid; L-alpha-Aminoindole-3-propionic acid; L-alpha-amino-3-indolepropionic acid; Alpha-Amino-beta-(3-indolyl)-propionic acid; Alpha-amino-beta-(3-indolyl)-pr opionic acid; Propionic acid, 2-amino-3-indol-3-yl; S(-)-1-alpha-Aminoindole-3-propionic acid; (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid; (L)-TRYPTOPHAN; (S)-(-)-2-Amino-3-(3-indolyl)propionic Acid; (S)-(-)-Tryptopha n; (S)-(-)-Tryptophan; (S)-2-Amino-3-(1H-indol-3-yl)propanoic acid; (S)-2-Amino-3-(3-indolyl)propionic acid; (S)-Tryptophan; (S)-a-Amino-1H-indole-3-propanoic acid; (S)-a-Amino-b-indolepropionic acid; (S)-a-Aminoindole-3-propionic acid; (S)-alpha-Amino-1H-indole-3-propanoic acid; (S)-alpha-Amino-beta-indolepropionic acid; (S)-alpha-Aminoindole-3-propionic acid; (S)-alpha-amino-beta-(3-indolyl)-propionic acid; 1-beta-3-Indolylalanine; 151A3008-4CFE-40C9-AC0B-467EF0CB50EA; 1H-Indole-3-alanine; 1H-Indole-3-alanine (VAN); 1beta-3-Indolylalanine; 2-Amino-3-(lH-indol-3-yl)-propanoic acid; 2-Amino-3-indolylpropanoic acid; 2-amino-3-indol-3-ylpropionic acid; 3-Indol-3-ylalanine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Depression [ICD-11: 6A70-6A7Z; ICD-9: 311] | Approved | [1], [2] | |
| Therapeutic Class |
Antidepressants
|
|||
| Structure |
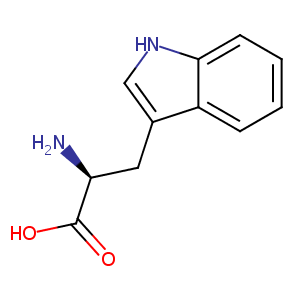 |
Download2D MOL |
||
| Formula |
C11H12N2O2
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=CN2)CC(C(=O)O)N
|
|||
| InChI |
1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
|
|||
| InChIKey |
QIVBCDIJIAJPQS-VIFPVBQESA-N
|
|||
| CAS Number |
CAS 73-22-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3378, 605298, 820596, 821032, 822330, 822480, 823942, 826461, 830210, 834088, 841615, 854680, 855044, 3134836, 6435732, 6436701, 7847088, 7888703, 7890877, 7980851, 8026883, 8144649, 8149576, 8153976, 10322924, 10534147, 11109884, 11111851, 11335632, 11360871, 11461843, 11528964, 14710264, 14710293, 14916602, 15195597, 17405739, 24276916, 24277675, 24278135, 24714971, 24770171, 24844224, 24889916, 24900200, 24900575, 25622645, 26703933, 26711994, 26712808
|
|||
| ChEBI ID |
CHEBI:16828
|
|||
| SuperDrug ATC ID |
N06AX02
|
|||
| SuperDrug CAS ID |
cas=000073223
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [3], [4] | |||
| Resulting Metabolite | Indole-3-propionic acid, tryptamine; indole | |||
| Metabolic Effect | Increase toxicity | |||
| Description | Tryptophan can be metabolized to Indole-3-propionic acid, tryptamine and indole by gut microbiota, which results in the increase of the drug's toxicity. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Indoleamine 2,3-dioxygenase 1 (IDO1) | Target Info | Binder | [5] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation | ||||
| L-kynurenine degradation | ||||
| Tryptophan degradation to 2-amino-3-carboxymuconate semialdehyde | ||||
| NAD de novo biosynthesis | ||||
| KEGG Pathway | Tryptophan metabolism | |||
| Metabolic pathways | ||||
| African trypanosomiasis | ||||
| NetPath Pathway | TSLP Signaling Pathway | |||
| IL5 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Tryptophan Metabolism | |||
| Reactome | Tryptophan catabolism | |||
| WikiPathways | Tryptophan metabolism | |||
| Metabolism of amino acids and derivatives | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 717). | |||
| REF 2 | Drug information of L-Tryptophan, 2008. eduDrugs. | |||
| REF 3 | Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017 Jun 23;356(6344):eaag2770. | |||
| REF 4 | Drug pharmacomicrobiomics and toxicomicrobiomics: from scattered reports to systematic studies of drug-microbiome interactions. Expert Opin Drug Metab Toxicol. 2018 Oct;14(10):1043-1055. | |||
| REF 5 | Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry. 2006 Jul 18;45(28):8527-38. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

