Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05FTJ
|
|||
| Former ID |
DAP000779
|
|||
| Drug Name |
Mefenamic acid
|
|||
| Synonyms |
ApoMefenamic; Bonabol; Contraflam; Coslan; Dysman; Lysalgo; Mefac; Mefacit; Mefedolo; Mefenacid;Mefenamate; Mefenaminsaeure; Mefic; Mycasaal; Namphen; NuMefenamic; Parkemed; Pinalgesic; Ponalar; Ponalgic; Ponmel; Ponstan; Ponstel; Ponstil; Ponstyl; Ponsyl; Pontal; Rolan; Tanston; Vialidon; APS Brand of Mefenamic Acid; Acide mefenamique; Acide mefenamique [French]; Acido mefenamico; Acidum mefenamicum; Antigen Brand of Mefenamic Acid; Apo Mefenamic; Apotex Brand of Mefenamic Acid; Ashbourne Brand of Mefenamic Acid; Chemidex Brand of Mefenamic Acid; Clonmel Brand of Mefenamic Acid; Elan Brand of Mefenamic Acid; Farmasierra Brand of Mefenamic Acid; First Horizon Brand of Mefenamic Acid; Godecke Brand of Mefenamic Acid; Mefanamic acid; Mefenaminic Acid; Mefenaminsaeure [German]; Mephenamic acid; Mephenaminic acid; Methenamic acid; Nu Mefenamic; Nu Pharm Brand of Mefenamic Acid; PMS Mefenamic Acid; Parke Davis Brand of Mefenamic Acid; Pfizer Brand of Mefenamic Acid; Pharmascience Brand of Mefenamic Acid; Pinewood Brand of Mefenamic Acid; Ponstan forte; Rowa Brand of Mefenamic Acid; Tamany Bonsan; Warner Lambert Brand of Mefenamic Acid; CL 473; CN 35355; HL 1; ID8; INF 3355; M1782; AGN-1255; Ac. mefenamico; Ac. mefenamico [Italian]; Acid, Mefenamic; Acid, Mefenaminic; Acide mefenamique [INN-French]; Acido mefenamico [INN-Spanish]; Acidum mefenamicum [INN-Latin]; Apo-Mefenamic; Bafameritin-M; Bafhameritin-M; CN-35355; Dyfenamic (TN); F0850-6853; Forte, Ponstan; INF-3355; In-M; Mafepain (TN); Meftal (TN); Mephadolor (TN); Nu-Mefenamic; Nu-Pharm Brand of Mefenamic Acid; PMS-Mefenamic Acid; Parkemed (TN); Ponstal (TN); Ponstan (TN); Ponstel (TN); Potarlon (TN); Warner-Lambert Brand of Mefenamic Acid; Mefenamic acid (JP15/USP/INN); Mefenamic acid [USAN:INN:BAN:JAN]; N-2,3-Xylylanthranilic acid; N-(2,3-Dimethylphenyl)anthranilic acid; N-(2,3-Xylyl)anthranilic acid; N-(2,3-Xylyl)-2-aminobenzoic acid; 2-((2,3-Dimethylphenyl)amino)benzoic acid; 2-(2,3-Dimethylanilino)benzoic acid; 2-(2,3-Xylidino)benzoic Acid; 2-(2,3-dimethylphenylamino)benzoic acid; 2-[(2,3-dimethylphenyl)amino]benzoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Dysmenorrhea [ICD-11: GA34.3] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Antiinflammatory Agents
|
|||
| Structure |
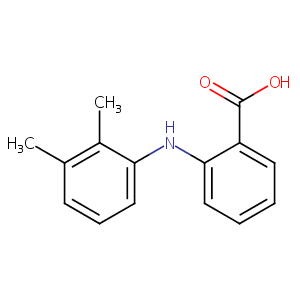 |
Download2D MOL |
||
| Formula |
C15H15NO2
|
|||
| Canonical SMILES |
CC1=C(C(=CC=C1)NC2=CC=CC=C2C(=O)O)C
|
|||
| InChI |
1S/C15H15NO2/c1-10-6-5-9-13(11(10)2)16-14-8-4-3-7-12(14)15(17)18/h3-9,16H,1-2H3,(H,17,18)
|
|||
| InChIKey |
HYYBABOKPJLUIN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 61-68-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5243, 399652, 798379, 840129, 855723, 4906250, 7847219, 7872085, 7979881, 8149703, 8152534, 10321491, 10530006, 11112163, 11335473, 11360712, 11363068, 11365630, 11368192, 11372043, 11374868, 11376354, 11461684, 11466082, 11467202, 11485737, 11485864, 11489757, 11490897, 11493049, 11493988, 14847469, 24424559, 24715034, 24896815, 26512268, 26612162, 26679725, 26746974, 26746975, 26751493, 29223155, 46505405, 47365092, 47440151, 47440152, 47662184, 47662185, 48035004, 48184900
|
|||
| ChEBI ID |
CHEBI:6717
|
|||
| ADReCS Drug ID | BADD_D01368 | |||
| SuperDrug ATC ID |
M01AG01
|
|||
| SuperDrug CAS ID |
cas=000061687
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [4] | |||
| Description | Mefenamic acid can be metabolized by gut microbiota. | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2593). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 015034. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Biodegradability of fluoxetine, mefenamic acid, and metoprolol using different microbial consortiums. Environ Sci Pollut Res Int. 2017 Mar;24(7):6779-6793. | |||
| REF 5 | Systematic pharmacological approach to the characterization of NSAIDs. Prostaglandins Leukot Essent Fatty Acids. 1998 Jul;59(1):55-62. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

