Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05KNG
|
|||
| Former ID |
DIB011063
|
|||
| Drug Name |
ALB-109564(a)
|
|||
| Synonyms |
ALB-109564; Vinca alkaloid analogs (cancer), Albany Molecular Research
Click to Show/Hide
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [1] | |
| Company |
Bessor-brightwaters pharma
|
|||
| Structure |
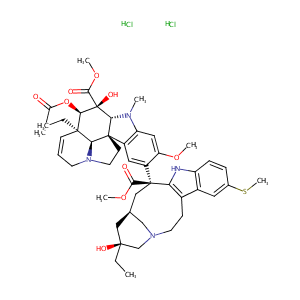 |
Download2D MOL |
||
| Formula |
C47H62Cl2N4O9S
|
|||
| Canonical SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=C(N3)C=CC(=C4)SC)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O.Cl.Cl
|
|||
| InChI |
1S/C47H60N4O9S.2ClH/c1-9-43(55)23-28-24-46(41(53)58-6,37-30(14-18-50(25-28)26-43)31-20-29(61-8)12-13-34(31)48-37)33-21-32-35(22-36(33)57-5)49(4)39-45(32)16-19-51-17-11-15-44(10-2,38(45)51)40(60-27(3)52)47(39,56)42(54)59-7;;/h11-13,15,20-22,28,38-40,48,55-56H,9-10,14,16-19,23-26H2,1-8H3;2*1H/t28-,38-,39+,40+,43-,44+,45+,46-,47-;;/m0../s1
|
|||
| InChIKey |
MTABGSVEFKMZFG-USKOTZBXSA-N
|
|||
| CAS Number |
CAS 1300114-12-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Tubulin beta (TUBB) | Target Info | Modulator | [2] |
| KEGG Pathway | Phagosome | |||
| Gap junction | ||||
| Pathogenic Escherichia coli infection | ||||
| NetPath Pathway | FSH Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| EGFR1 Signaling Pathway | ||||
| Panther Pathway | Cytoskeletal regulation by Rho GTPase | |||
| Huntington disease | ||||
| Reactome | Regulation of PLK1 Activity at G2/M Transition | |||
| Loss of Nlp from mitotic centrosomes | ||||
| Recruitment of mitotic centrosome proteins and complexes | ||||
| Loss of proteins required for interphase microtubule organization?from the centrosome | ||||
| Anchoring of the basal body to the plasma membrane | ||||
| WikiPathways | Parkin-Ubiquitin Proteasomal System pathway | |||
| Pathogenic Escherichia coli infection | ||||
| Mitotic G2-G2/M phases | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00724100) A Trial of ALB 109564(a) in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||
| REF 2 | 2010 ASCO Annual Meeting | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

