Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05XLO
|
|||
| Former ID |
DCL000134
|
|||
| Drug Name |
INCB13739
|
|||
| Synonyms |
Phenylarsine oxide; phenylarsine oxide; Oxophenylarsine; 637-03-6; Arzene; Phenylarsenoxide; Arsenosobenzene; ARSINE, OXOPHENYL-; Phenyl arsine oxide; Benzene, arsenoso-; arsorosobenzene; Fenylarsinoxid; Caswell No 060; Fenylarsinoxid [Czech]; C6H5AsO; Phenyl arsenoxide; UNII-0HUR2WY345; PAO; EINECS 211-275-3; NSC 42470; EPA Pesticide Chemical Code 007101; BRN 2935227; 0HUR2WY345; CHEBI:75253; MFCD00001990; Phenylarsine oxide, 97%; Phenylarsine Oxide Solution; oxo(phenyl)arsan; Phenylarsinoxyd; oxo(phenyl)arsine; oxo(phenyl)arsane; PhAsO; phenylarsenious
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 2a | [1] | |
| Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Phase 2 | [1] | ||
| Company |
Incyte
|
|||
| Structure |
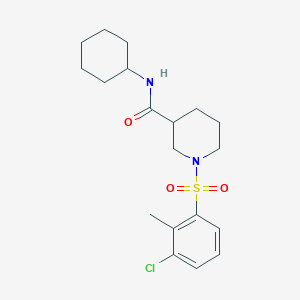 |
Download2D MOL |
||
| Formula |
C19H27ClN2O3S
|
|||
| Canonical SMILES |
CC1=C(C=CC=C1Cl)S(=O)(=O)N2CCCC(C2)C(=O)NC3CCCCC3
|
|||
| InChI |
1S/C19H27ClN2O3S/c1-14-17(20)10-5-11-18(14)26(24,25)22-12-6-7-15(13-22)19(23)21-16-8-3-2-4-9-16/h5,10-11,15-16H,2-4,6-9,12-13H2,1H3,(H,21,23)
|
|||
| InChIKey |
ODRPEKCTTLECBX-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
97034, 630901, 3137510, 6752686, 8152934, 15321835, 24898387, 26759339, 29223862, 49834324, 49871838, 53787614, 57322444, 78628055, 85088620, 87574393, 88833008, 103639549, 103843612, 104307400, 117357194, 134338816, 134340358, 134978308, 135562904, 137006306, 142276159, 144211636, 151979814, 162091628, 163687514, 163835449, 176329210, 179148757, 198957874, 201506311, 204361893, 212344127, 226523248, 226684484, 241114067, 249626677, 250033911, 252503034
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Corticosteroid 11-beta-dehydrogenase 1 (HSD11B1) | Target Info | Inhibitor | [2] |
| Protein tyrosine phosphatase (PTP) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Steroid hormone biosynthesis | |||
| Metabolism of xenobiotics by cytochrome P450 | ||||
| Metabolic pathways | ||||
| Chemical carcinogenesis | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| FSH Signaling Pathway | ||||
| Pathwhiz Pathway | Steroidogenesis | |||
| Reactome | Glucocorticoid biosynthesis | |||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| Metabolism of steroid hormones and vitamin D | ||||
| Glucocorticoid & Mineralcorticoid Metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00698230) Safety and Efficacy of INCB013739 Plus Metformin Compared to Metformin Alone on Glycemic Control in Type 2 Diabetics. U.S. National Institutes of Health. | |||
| REF 2 | Incyte's Selective Oral Inhibitor Of 11beta-HSD1 Demonstrates Improvements In Insulin Sensitivity And Lowers Cholesterol Levels In Type 2 Diabetics. Incyte. 2008. | |||
| REF 3 | Induction of endothelial cell surface adhesion molecules by tumor necrosis factor is blocked by protein tyrosine phosphatase inhibitors: role of the nuclear transcription factor NF-kappa B. Eur J Immunol. 1997 Sep;27(9):2172-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

