Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06EMM
|
|||
| Former ID |
DIB010260
|
|||
| Drug Name |
CERC-301
|
|||
| Indication | Major depressive disorder [ICD-11: 6A70.3; ICD-10: F32.2] | Phase 2 | [1] | |
| Company |
Cerecor
|
|||
| Structure |
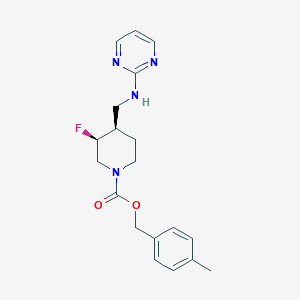 |
Download2D MOL |
||
| Formula |
C19H23FN4O2
|
|||
| Canonical SMILES |
CC1=CC=C(C=C1)COC(=O)N2CCC(C(C2)F)CNC3=NC=CC=N3
|
|||
| InChI |
1S/C19H23FN4O2/c1-14-3-5-15(6-4-14)13-26-19(25)24-10-7-16(17(20)12-24)11-23-18-21-8-2-9-22-18/h2-6,8-9,16-17H,7,10-13H2,1H3,(H,21,22,23)/t16-,17-/m1/s1
|
|||
| InChIKey |
RECBFDWSXWAXHY-IAGOWNOFSA-N
|
|||
| CAS Number |
CAS 808733-05-3
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02459236) A Study of Intermittent Doses of CERC-301 in MDD. | |||
| REF 2 | Inhibition of in vivo [(3)H]MK-801 binding by NMDA receptor open channel blockers and GluN2B antagonists in rats and mice. Eur J Pharmacol. 2015 Nov 5;766:1-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

