Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06MAJ
|
|||
| Former ID |
DAP001403
|
|||
| Drug Name |
Mizolastine
|
|||
| Synonyms |
Mistalin; Mistamine; Mizolastina; Mizolastinum; Mizolen; Mizollen; Zolim; Zolistam; Zolistan; Allphar brand of mizolastine; Galderma brand of mizolastine; Mizolastine [INN]; Novag brand of mizolastine; Sanofi Synthelabo brand of mizolastine; Schwarz brand of mizolastine; MKC-431; Mizolastina [INN-Spanish]; Mizolastinum [INN-Latin]; Mizollen (TN); SL 85.0324; Mizolastine (JAN/INN); SL-85.0324; 2-((1-(1-(p-Fluorobenzyl)-2-benzimidazolyl)-4-piperidyl)methylamino)-4(3H)-pyrimidinone; 2-[[1-[1-[(4-fluorophenyl)methyl]benzimidazol-2-yl]piperidin-4-yl]-methylamino]-1H-pyrimidin-6-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergic rhinitis [ICD-11: CA08.0] | Approved | [1] | |
| Therapeutic Class |
Antiallergic Agents
|
|||
| Structure |
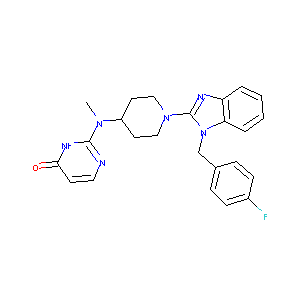 |
Download2D MOL |
||
| Formula |
C24H25FN6O
|
|||
| Canonical SMILES |
CN(C1CCN(CC1)C2=NC3=CC=CC=C3N2CC4=CC=C(C=C4)F)C5=NC=CC(=O)N5
|
|||
| InChI |
1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32)
|
|||
| InChIKey |
PVLJETXTTWAYEW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 108612-45-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7848180, 8190025, 12014077, 14783219, 14856657, 43122645, 49846691, 50036887, 50125752, 50713968, 57315819, 81041146, 89736146, 92714282, 96099817, 103305895, 104334988, 109692956, 118843605, 125337563, 126624261, 126655525, 126666541, 127327796, 127327797, 127327798, 127327799, 127327800, 127327801, 127327802, 128973914, 131294183, 134338611, 135024365, 135692425, 137246302, 137267724, 138213095, 142886695, 144206216, 160828705, 162178211, 163093678, 163390269, 163842321, 164230570, 164812773, 170465604, 172914175, 174526115
|
|||
| ChEBI ID |
CHEBI:31857
|
|||
| SuperDrug ATC ID |
R06AX25
|
|||
| SuperDrug CAS ID |
cas=108612459
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Population pharmacokinetic analysis and optimization of the experimental design for mizolastine solution in children. J Pharmacokinet Pharmacodyn. 2001 Jun;28(3):299-319. | |||
| REF 2 | Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms. Clin Exp Allergy. 2008 Jun;38(6):947-56. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

