Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06ZWL
|
|||
| Former ID |
DNC000194
|
|||
| Drug Name |
Amdoxovir
|
|||
| Synonyms |
Amdoxovir; DAPD; 145514-04-1; DAPD cpd; UNII-54I81H0M9C; (-)-DAPD; beta-D-2,6-Diaminopurine-dioxolane; 54I81H0M9C; [(2R,4R)-4-(2,6-diaminopurin-9-yl)-1,3-dioxolan-2-yl]methanol; (2R-cis)-4-(2,6-Diamino-9H-purin-9-yl)-1,3-dioxolane-2-methanol; (2R,4R)-4-(2,6-Diamino-9H-purin-9-yl)-1,3-dioxolane-2-methanol; (-)-(2R,4R)-2-Amino-(2-(hydroxymethyl)-1,3-dioxolan-4-yl)adenine; 1,3-Dioxolane-2-methanol, 4-(2,6-diamino-9H-purin-9-yl)-, (2R,4R)-; 1,3-Dioxolane-2-methanol, 4-(2,6-diamino-9H-purin-9-yl)-, (2R-cis)-; Amdoxovir [USAN]
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hepatitis B virus infection [ICD-11: 1E51.0; ICD-10: B18.1] | Phase 2 | [1] | |
| Structure |
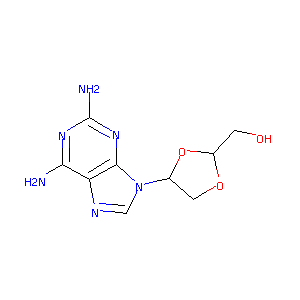 |
Download2D MOL |
||
| Formula |
C9H12N6O3
|
|||
| Canonical SMILES |
C1C(OC(O1)CO)N2C=NC3=C(N=C(N=C32)N)N
|
|||
| InChI |
1S/C9H12N6O3/c10-7-6-8(14-9(11)13-7)15(3-12-6)4-2-17-5(1-16)18-4/h3-5,16H,1-2H2,(H4,10,11,13,14)/t4-,5-/m1/s1
|
|||
| InChIKey |
RLAHNGKRJJEIJL-RFZPGFLSSA-N
|
|||
| CAS Number |
CAS 145514-04-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| SuperDrug ATC ID |
J05AB04
|
|||
| SuperDrug CAS ID |
cas=036791045
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Reverse transcriptase (HIV RT) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01738555) A Safety and Efficacy Study of Amdoxovir in HIV-1 Treatment-experienced Subjects. U.S. National Institutes of Health. | |||
| REF 2 | Amdoxovir versus placebo with enfuvirtide plus optimized background therapy for HIV-1-infected subjects failing current therapy (AACTG A5118). Antivir Ther. 2006;11(5):619-23. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

