Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07GBV
|
|||
| Former ID |
DNC003983
|
|||
| Drug Name |
3-(1-Phenethyl-piperidin-4-yl)-2-phenyl-1H-indole
|
|||
| Synonyms |
CHEMBL40584; 3-(1-Phenethyl-piperidin-4-yl)-2-phenyl-1H-indole; SCHEMBL7948313; BDBM50095049; L018755; 3-(1-phenethylpiperidin-4-yl)-2-phenyl-1H-indole
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
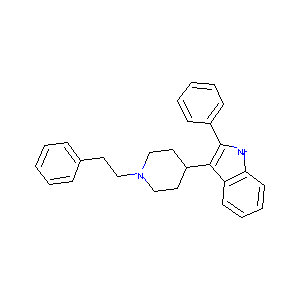 |
Download2D MOL |
||
| Formula |
C27H28N2
|
|||
| Canonical SMILES |
C1CN(CCC1C2=C(NC3=CC=CC=C32)C4=CC=CC=C4)CCC5=CC=CC=C5
|
|||
| InChI |
1S/C27H28N2/c1-3-9-21(10-4-1)15-18-29-19-16-22(17-20-29)26-24-13-7-8-14-25(24)28-27(26)23-11-5-2-6-12-23/h1-14,22,28H,15-20H2
|
|||
| InChIKey |
MONKRYYJEIHBRU-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 2A receptor (HTR2A) | Target Info | Inhibitor | [1] |
| Voltage-gated potassium channel Kv11.1 (KCNH2) | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Gap junction | ||||
| Serotonergic synapse | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | 5HT2 type receptor mediated signaling pathway | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Voltage gated Potassium channels | |||
| Serotonin receptors | ||||
| G alpha (q) signalling events | ||||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Hematopoietic Stem Cell Differentiation | ||||
| Potassium Channels | ||||
| Serotonin Receptor 2 and STAT3 Signaling | ||||
| Serotonin Receptor 2 and ELK-SRF/GATA4 signaling | ||||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 3-(4-Piperidinyl)- and 3-(8-aza-bicyclo[3.2.1]oct-3-yl)-2-phenyl-1H-indoles as bioavailable h5-HT2A antagonists. Bioorg Med Chem Lett. 2000 Dec 18;10(24):2701-3. | |||
| REF 2 | 3-(4-Fluoropiperidin-3-yl)-2-phenylindoles as high affinity, selective, and orally bioavailable h5-HT(2A) receptor antagonists. J Med Chem. 2001 May 10;44(10):1603-14. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

