Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07ZNI
|
|||
| Former ID |
DIB007872
|
|||
| Drug Name |
STX-140
|
|||
| Synonyms |
(9beta,13alpha,14beta,17alpha)-2-Methoxyestra-1,3,5(10)-Triene-3,17-Diyl Disulfamate; STX140; CHEMBL218382; 2-MeOE2bisMATE; 2-methoxyestradiol-3,17-O,O-bis-sulfamate; STX 140; 2-MeOEbisMATE; 2gd8; 2-methoxyestradiol 3,17-O,O-bis-sulfamate; 2-MbM; 2-methoxyestradiol-3,17-O,O-bis(sulfamate); SCHEMBL446846; BDBM50200936; DB08416; (9beta,17beta)-2-methoxyestra-1,3,5(10)-triene-3,17-diyl disulfamate; Estra-1,3,5(10)-triene-3,17-diol, 2-methoxy-, disulfamate, (17beta)-; 401600-86-0; Angiomates; Estrone sulphatase inhibitors, Ipsen; STX-213; STX-641; Steroid sulfatase inhibitors, Sterix Ltd; 2-MeOE2-bis MATE; 2-MeSEMATE; 2-ethyl-estradiol bis-sulfamate; 2-methoxyestradiol, Sterix; 2-methoxyestradiol-bis-sulfamate; 2-methoxyestradiol-bis-sulfamate, Sterix
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Osteoporosis [ICD-11: FB83.0; ICD-10: M85.8] | Phase 2 | [1] | |
| Company |
EntreMed
|
|||
| Structure |
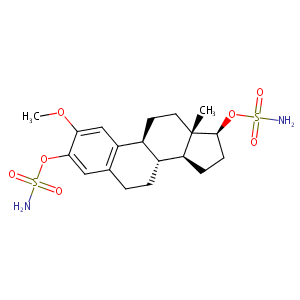 |
Download2D MOL |
||
| Formula |
C19H28N2O7S2
|
|||
| Canonical SMILES |
CC12CCC3C(C1CCC2OS(=O)(=O)N)CCC4=CC(=C(C=C34)OC)OS(=O)(=O)N
|
|||
| InChI |
1S/C19H28N2O7S2/c1-19-8-7-12-13(15(19)5-6-18(19)28-30(21,24)25)4-3-11-9-17(27-29(20,22)23)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,20,22,23)(H2,21,24,25)/t12-,13+,15-,18-,19-/m0/s1
|
|||
| InChIKey |
AQSNIXKAKUZPSI-SSTWWWIQSA-N
|
|||
| CAS Number |
CAS 401600-86-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Carbonic anhydrase II (CA-II) | Target Info | Inhibitor | [2] |
| Steryl-sulfatase (STS) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Steroid hormone biosynthesis | |||
| Nitrogen metabolism | ||||
| Proximal tubule bicarbonate reclamation | ||||
| Collecting duct acid secretion | ||||
| Gastric acid secretion | ||||
| Pancreatic secretion | ||||
| Bile secretion | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| EGFR1 Signaling Pathway | ||||
| Pathwhiz Pathway | Androgen and Estrogen Metabolism | |||
| Reactome | Glycosphingolipid metabolism | |||
| Erythrocytes take up carbon dioxide and release oxygen | ||||
| Erythrocytes take up oxygen and release carbon dioxide | ||||
| Reversible hydration of carbon dioxide | ||||
| WikiPathways | Estrogen metabolism | |||
| Vitamin D Receptor Pathway | ||||
| Sphingolipid metabolism | ||||
| Reversible Hydration of Carbon Dioxide | ||||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | ||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00592579) A Phase 2 Study With Panzem in Patients With Relapsed or Plateau Phase Multiple Myeloma. U.S. National Institutes of Health. | |||
| REF 2 | Structure-activity relationships of C-17 cyano-substituted estratrienes as anticancer agents. J Med Chem. 2008 Mar 13;51(5):1295-308. | |||
| REF 3 | 2-substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J Med Chem. 2006 Dec 28;49(26):7683-96. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

