Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08HGY
|
|||
| Former ID |
DIB005659
|
|||
| Drug Name |
Naronapride
|
|||
| Synonyms |
Naronapride dihydrochloride; ATI-7500; ATI-7505; Cisapride analog, ARYx Therapeutics; ATI-7000 series, ARYx Therapeutics; Cisapride analog, Procter & Gamble; ATI-7000 series, Procter & Gamble; 5-HT 4 receptor agonist (oral, gastroesophageal reflux/irritable bowel syndrome/gastric motilitydisorder/constipation/dyspepsia), ARYx Therapeutics/Procter & Gamble Pharmaceuticals Inc
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Gastroesophageal reflux disease [ICD-11: DA22.Z] | Discontinued in Phase 2 | [1] | |
| Company |
ARYx Therapeutics
|
|||
| Structure |
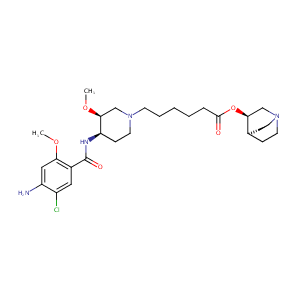 |
Download2D MOL |
||
| Formula |
C27H41ClN4O5
|
|||
| Canonical SMILES |
COC1CN(CCC1NC(=O)C2=CC(=C(C=C2OC)N)Cl)CCCCCC(=O)OC3CN4CCC3CC4
|
|||
| InChI |
1S/C27H41ClN4O5/c1-35-23-15-21(29)20(28)14-19(23)27(34)30-22-9-13-31(17-25(22)36-2)10-5-3-4-6-26(33)37-24-16-32-11-7-18(24)8-12-32/h14-15,18,22,24-25H,3-13,16-17,29H2,1-2H3,(H,30,34)/t22-,24+,25+/m1/s1
|
|||
| InChIKey |
VGDDOIZXGFJDRC-VJTSUQJLSA-N
|
|||
| CAS Number |
CAS 860174-12-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 4 receptor (HTR4) | Target Info | Agonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT4 type receptor mediated signaling pathway | ||||
| Pathwhiz Pathway | Excitatory Neural Signalling Through 5-HTR 4 and Serotonin | |||
| Reactome | Serotonin receptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Serotonin Receptor 4/6/7 and NR3C Signaling | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019926) | |||
| REF 2 | Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012 Apr;35(7):745-67. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

