Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08LDS
|
|||
| Former ID |
DNC012213
|
|||
| Drug Name |
LUBELUZOLE
|
|||
| Synonyms |
Lubeluzole; Prosynap; 144665-07-6; UNII-V2SIB71583; CHEMBL281724; V2SIB71583; R 87926; R-87926; (2S)-1-[4-[1,3-benzothiazol-2-yl(methyl)amino]piperidin-1-yl]-3-(3,4-difluorophenoxy)propan-2-ol; (+)-(S)-4-(2-Benzothiazolylmethylamino)-alpha-((3,4-difluorophenoxy)methyl)-1-piperidineethanol; 1-Piperidineethanol, 4-(2-benzothiazolylmethylamino)-alpha-((3,4-difluorophenoxy)methyl)-, (S)-; Lubeluzol; Lubeluzole [USAN:INN:BAN]; C22H25F2N3O2S; Prosynap (TN); R-91154; AC1Q4ONU; Lubeluzole (USAN/INN); SCHEMBL159725; Lubeluzole [USAN:B
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Neurological disorder [ICD-11: 6B60] | Discontinued in Preregistration | [1] | |
| Structure |
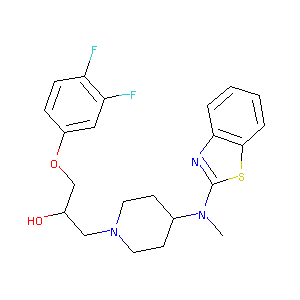 |
Download2D MOL |
||
| Formula |
C22H25F2N3O2S
|
|||
| Canonical SMILES |
CN(C1CCN(CC1)CC(COC2=CC(=C(C=C2)F)F)O)C3=NC4=CC=CC=C4S3
|
|||
| InChI |
1S/C22H25F2N3O2S/c1-26(22-25-20-4-2-3-5-21(20)30-22)15-8-10-27(11-9-15)13-16(28)14-29-17-6-7-18(23)19(24)12-17/h2-7,12,15-16,28H,8-11,13-14H2,1H3/t16-/m0/s1
|
|||
| InChIKey |
OZFSWVOEXHGDES-INIZCTEOSA-N
|
|||
| CAS Number |
CAS 144665-07-6
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:135703
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Sodium channel unspecific (NaC) | Target Info | Inhibitor | [2] |
| Voltage-gated sodium channel alpha Nav1.5 (SCN5A) | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Dopaminergic synapse | |||
| Adrenergic signaling in cardiomyocytes | ||||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Cardiac Progenitor Differentiation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005388) | |||
| REF 2 | Medicinal chemistry of neuronal voltage-gated sodium channel blockers. J Med Chem. 2001 Jan 18;44(2):115-37. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

