Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08VXO
|
|||
| Former ID |
DAP001066
|
|||
| Drug Name |
Brompheniramine
|
|||
| Synonyms |
Bromfed; Bromfenex; Bromfeniramina; Brotane; Parabromdylamine; BROMFED-DM; Bromfeniramina [INN-Spanish]; Brompheniramine (INN); Brompheniramine [INN:BAN]; Brompheniraminum [INN-Latin]; Brotane (TN); DIMETANE-DX; P-Bromdylamine; Para-Bromdylamine; Brompheniramine Maleate (1:1); [3-(4-Bromophenyl)-3-(2-pyridyl)propyl]dimethylamine; Gamma-(4-Bromophenyl)-N,N-dimethyl-2-pyridinepropanamine; 1-(p-Bromophenyl)-1-(2-pyridyl)-3-dimethylaminopropane; 2-(p-Bromo-alpha-(2-dimethylaminoethyl)benzyl)pyridine; 3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine; 3-(4-bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine; 3-(4-bromophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine; 3-(p-Bromophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergic rhinitis [ICD-11: CA08.0] | Approved | [1], [2] | |
| Therapeutic Class |
Antiallergic Agents
|
|||
| Company |
Alpharma Us Pharmaceuticals Division
|
|||
| Structure |
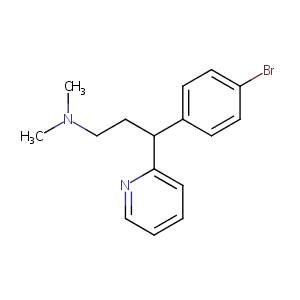 |
Download2D MOL |
||
| Formula |
C16H19BrN2
|
|||
| Canonical SMILES |
CN(C)CCC(C1=CC=C(C=C1)Br)C2=CC=CC=N2
|
|||
| InChI |
1S/C16H19BrN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3
|
|||
| InChIKey |
ZDIGNSYAACHWNL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 86-22-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9075, 615216, 6677389, 7978822, 8154449, 10537061, 11342021, 11362204, 11364576, 11367138, 11369700, 11371960, 11374692, 11377862, 11466503, 11467623, 11485355, 11486285, 11487606, 11489426, 11490796, 11492921, 11495496, 15396259, 29225774, 46508137, 47248568, 47397182, 47397183, 47472595, 47472596, 47546820, 47768508, 47991967, 47991968, 48291421, 48415651, 49698955, 50031272, 50065321, 50070760, 50105249, 50892257, 51091865, 85209729, 85788883, 90341200, 92714616, 92729935, 96099913
|
|||
| ChEBI ID |
CHEBI:3183
|
|||
| SuperDrug ATC ID |
R06AB01
|
|||
| SuperDrug CAS ID |
cas=000086226
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7133). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 083821. | |||
| REF 3 | Histamine-induced venodilation in human beings involves both H1 and H2 receptor subtypes. J Allergy Clin Immunol. 1994 Mar;93(3):606-14. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

