Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08ZEB
|
|||
| Former ID |
DAP000952
|
|||
| Drug Name |
Chlorzoxazone
|
|||
| Synonyms |
Biomioran; CLW; Chloroxazone; Chlorsoxazone; Chlorzoxane; Chlorzoxazon; Chlorzoxazona; Chlorzoxazonum; Clorzoxazona; Escoflex; Klorzoxazon; Mioran; Miotran; Myoflexin; Myoflexine; Neoflex; Nyoflex; Paraflex; Parafon; Pathorysin; Relaxazone; Remofleks; Remular; Solaxin; Component of Parafon Forte; McNeil Brand of Chlorzoxazone; Ortho Brand of Chlorzoxazone; Parafon Forte; Parafon Forte DSC; Strifon Forte Dsc; C 4397; Chlorzoxazonum [INN-Latin]; Clorzoxazona [INN-Spanish]; EZE-DS; Muscol (TN); Paraflex (TN); Parafon Forte (TN); Remular-S; Usaf ma-10; Chlorzoxazone [INN:BAN:JAN]; Chlorzoxazone (JAN/USP/INN); 2-Hydroxy-5-chlorobenzoxazole; 5-Chlorbenzoxazolin-2-on; 5-Chloro-1,3-benzoxazol-2(3H)-one; 5-Chloro-2(3H)-benzoxazolone; 5-Chloro-2-benzoxazolinone; 5-Chloro-2-benzoxazolol; 5-Chloro-2-benzoxazolone; 5-Chloro-2-hydroxybenzoxazole; 5-Chloro-3(H)-2-benzoxazolone; 5-Chlorobenzoksazolinon-2; 5-Chlorobenzoksazolinon-2 [Polish]; 5-Chlorobenzoksazolon-2; 5-Chlorobenzoksazolon-2 [Polish]; 5-Chlorobenzoxazol-2-one; 5-Chlorobenzoxazolidone; 5-Chlorobenzoxazolone; 5-chloro-1,3-benzoxazol-2-ol; 5-chloro-3H-1,3-benzoxazol-2-one; 5-chlorobenzoxazolin-2-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute pain [ICD-11: MG31; ICD-10: R52.0; ICD-9: 338,780] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Analgesics
|
|||
| Structure |
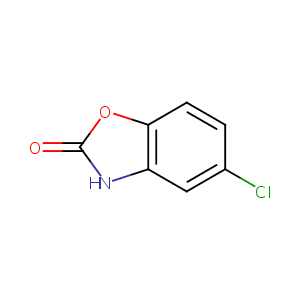 |
Download2D MOL |
||
| Formula |
C7H4ClNO2
|
|||
| Canonical SMILES |
C1=CC2=C(C=C1Cl)NC(=O)O2
|
|||
| InChI |
1S/C7H4ClNO2/c8-4-1-2-6-5(3-4)9-7(10)11-6/h1-3H,(H,9,10)
|
|||
| InChIKey |
TZFWDZFKRBELIQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 95-25-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10133, 87091, 826390, 826422, 3139748, 6867174, 7767972, 7847836, 7886649, 8139927, 8149257, 8151769, 10321163, 10538622, 11110945, 11110946, 11336070, 11361309, 11363042, 11365604, 11368166, 11371339, 11373953, 11376328, 11462281, 11466191, 11467311, 11484813, 11485682, 11488965, 11490166, 11492102, 11493962, 16935856, 17404816, 24277702, 26611661, 26679366, 26747469, 26747470, 29221889, 46507755, 47291196, 47662345, 47736550, 47810813, 47959818, 48035200, 48334567, 48334568
|
|||
| ChEBI ID |
CHEBI:3655
|
|||
| ADReCS Drug ID | BADD_D00449 | |||
| SuperDrug ATC ID |
M03BB03
|
|||
| SuperDrug CAS ID |
cas=000095250
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Calcium-activated potassium channel (KCN) | Target Info | Activator | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2322). | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040113. | |||
| REF 4 | Chlorzoxazone inhibits contraction of rat thoracic aorta. Eur J Pharmacol. 2006 Sep 18;545(2-3):161-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

