Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09CLP
|
|||
| Former ID |
DNC009797
|
|||
| Drug Name |
(L-)-S-adenosyl-L-homocysteine
|
|||
| Synonyms |
S-Adenosyl-L-homocysteine; S-adenosylhomocysteine; S-adenosyl-L-homocysteine; 979-92-0; AdoHcy; S-(5'-adenosyl)-L-homocysteine; adenosylhomocysteine; Formycinylhomocysteine; Adenosyl-L-homocysteine; S-(5'-deoxyadenosin-5'-yl)-L-homocysteine; 2-S-adenosyl-L-homocysteine; 5'-Deoxy-S-adenosyl-L-homocysteine; S-adenosyl-homocysteine; S-Adenosyl Homocysteine; L-S-Adenosylhomocysteine; L-Homocysteine, S-(5'-deoxyadenosin-5'-yl)-; adenosylhomo-cys; adenosyl-homo-cys; UNII-8K31Q2S66S; (S)-5'-(S)-(3-Amino-3-carboxypropyl)-5'-thioadenosine; BRN 5166233; SAH; S-Adenosylhomocysteine; S-Adenosyl-L-Homocysteine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2], [3] | |
| Structure |
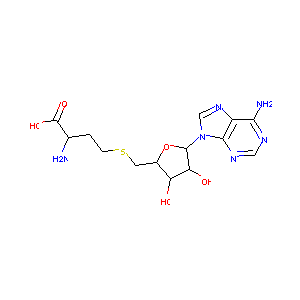 |
Download2D MOL |
||
| Formula |
C14H20N6O5S
|
|||
| Canonical SMILES |
C1=NC(=C2C(=N1)N(C=N2)C3C(C(C(O3)CSCCC(C(=O)O)N)O)O)N
|
|||
| InChI |
1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1
|
|||
| InChIKey |
ZJUKTBDSGOFHSH-WFMPWKQPSA-N
|
|||
| CAS Number |
CAS 979-92-0
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:16680
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA [cytosine-5]-methyltransferase (DNMT) | Target Info | Inhibitor | [3], [4] |
| DNA [cytosine-5]-methyltransferase 3B (DNMT3B) | Target Info | Inhibitor | [3], [4] | |
| Histamine N-methyltransferase (HNMT) | Target Info | Inhibitor | [1] | |
| BioCyc | Histamine degradation | |||
| KEGG Pathway | Histidine metabolism | |||
| Cysteine and methionine metabolism | ||||
| Metabolic pathways | ||||
| MicroRNAs in cancer | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Pathwhiz Pathway | Histidine Metabolism | |||
| Reactome | PRC2 methylates histones and DNA | |||
| NoRC negatively regulates rRNA expression | ||||
| DNA methylation | ||||
| WikiPathways | Methylation Pathways | |||
| Metapathway biotransformation | ||||
| Mesodermal Commitment Pathway | ||||
| Endoderm Differentiation | ||||
| Trans-sulfuration and one carbon metabolism | ||||
| One Carbon Metabolism | ||||
| miR-targeted genes in lymphocytes - TarBase | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5265). | |||
| REF 3 | Constrained (l-)-S-adenosyl-l-homocysteine (SAH) analogues as DNA methyltransferase inhibitors. Bioorg Med Chem Lett. 2009 May 15;19(10):2742-6. | |||
| REF 4 | Novel and selective DNA methyltransferase inhibitors: Docking-based virtual screening and experimental evaluation. Bioorg Med Chem. 2010 Jan 15;18(2):822-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

