Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09DLP
|
|||
| Former ID |
DIB002636
|
|||
| Drug Name |
Tramiprosate
|
|||
| Synonyms |
Alzhemed; Cerebril; Homotaurine; Vivimind; LU-02659; NC-531; NC-758; Tramiprosate (stroke), Neurochem; Tramiprosate (Alzheimer's disease), Neurochem; 3APS
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 3 | [1] | |
| Company |
Neurochem
|
|||
| Structure |
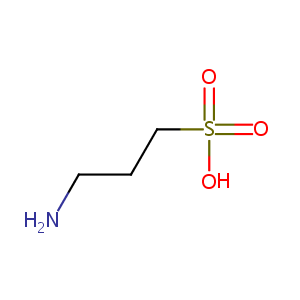 |
Download2D MOL |
||
| Formula |
C3H9NO3S
|
|||
| Canonical SMILES |
C(CN)CS(=O)(=O)O
|
|||
| InChI |
1S/C3H9NO3S/c4-2-1-3-8(5,6)7/h1-4H2,(H,5,6,7)
|
|||
| InChIKey |
SNKZJIOFVMKAOJ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 3687-18-1
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:1457
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00314912) Open-Label Extension of the Phase III Study With Tramiprosate (3APS) in Patients With Mild to Moderate Alzheimer's Disease. U.S. National Institutes of Health. | |||
| REF 2 | Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007 Apr;28(4):537-47. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

