Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09ELP
|
|||
| Former ID |
DNAP001691
|
|||
| Drug Name |
Clevidipine butyrate
|
|||
| Synonyms |
Clevidipine; Cleviprex; 167221-71-8; Clevelox; rac-Clevidipine; H 324/38; Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dime thyl-3,5-pyridinedicarboxylate; Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate; Cleviprex (TN); 3-((butyryloxy)methyl) 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; METHYL 5-{[(BUTANOYLOXY)METHOXY]CARBONYL}-4-(2,3-DICHLOROPHENYL)-2,6-DIMETHYL-1,4-DIHYDROPYRIDINE-3
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Approved | [1], [2] | |
| Company |
The Medicines Company
|
|||
| Structure |
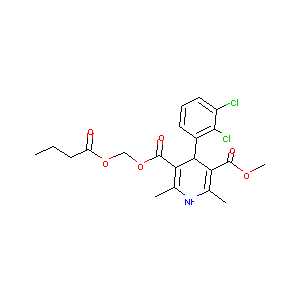 |
Download2D MOL |
||
| Formula |
C21H23Cl2NO6
|
|||
| Canonical SMILES |
CCCC(=O)OCOC(=O)C1=C(NC(=C(C1C2=C(C(=CC=C2)Cl)Cl)C(=O)OC)C)C
|
|||
| InChI |
1S/C21H23Cl2NO6/c1-5-7-15(25)29-10-30-21(27)17-12(3)24-11(2)16(20(26)28-4)18(17)13-8-6-9-14(22)19(13)23/h6,8-9,18,24H,5,7,10H2,1-4H3
|
|||
| InChIKey |
KPBZROQVTHLCDU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 167221-71-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:135738
|
|||
| ADReCS Drug ID | BADD_D00482 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Calcium channel unspecific (CaC) | Target Info | Modulator | [1], [2] |
| Voltage-gated L-type calcium channel (L-CaC) | Target Info | Inhibitor | [3] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | |||
| REF 2 | Clevidipine: a short-acting intravenous dihydropyridine calcium channel blocker for the management of hypertension. Pharmacotherapy. 2010 May;30(5):515-28. | |||
| REF 3 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

