Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09JVV
|
|||
| Former ID |
DIB000333
|
|||
| Drug Name |
Physostigmine
|
|||
| Synonyms |
Xalieve; Acetylcholinesterase inhibitor (dry mouth), Calabar; Physostigmine (local acting gel, xerostomia); Physostigmine (buccal, xerostomia), Calabar; Physostigmine (local acting gel, xerostomia), Calabar
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Xerostomia [ICD-11: DA02.1; ICD-10: R68, R68.2; ICD-9: 527.7] | Discontinued in Phase 2 | [1], [2] | |
| Company |
Calabar AB
|
|||
| Structure |
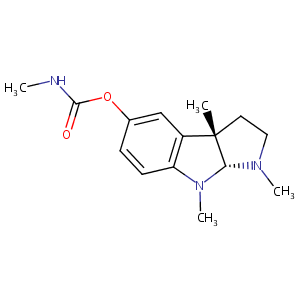 |
Download2D MOL |
||
| Formula |
C15H21N3O2
|
|||
| Canonical SMILES |
CC12CCN(C1N(C3=C2C=C(C=C3)OC(=O)NC)C)C
|
|||
| InChI |
1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1
|
|||
| InChIKey |
PIJVFDBKTWXHHD-HIFRSBDPSA-N
|
|||
| CAS Number |
CAS 57-47-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
8765, 89892, 7847264, 7979185, 8138132, 8153719, 11341652, 11361835, 11363810, 11364692, 11366372, 11367254, 11368934, 11369816, 11371789, 11371941, 11374571, 11375199, 11375666, 11377096, 11377979, 11466594, 11467714, 11484075, 11484510, 11485021, 11486307, 11487237, 11488146, 11488573, 11489099, 11490361, 11490716, 11492811, 11493445, 11493727, 11494730, 11495603, 11537599, 14848555, 15222048, 17405046, 24277867, 24869283, 25626763, 26612055, 26679946, 26753571, 26753572, 29224999
|
|||
| ChEBI ID |
CHEBI:27953
|
|||
| ADReCS Drug ID | BADD_D01768 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Acetylcholinesterase (AChE) | Target Info | Inhibitor | [3] |
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Cholinergic synapse | ||||
| Panther Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | ||||
| Pathwhiz Pathway | Phospholipid Biosynthesis | |||
| Pathway Interaction Database | ATF-2 transcription factor network | |||
| WikiPathways | Monoamine Transport | |||
| Biogenic Amine Synthesis | ||||
| Acetylcholine Synthesis | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6598). | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028816) | |||
| REF 3 | The effects of physostigmine on acetylcholinesterase activity of CSF plasma and brain. A comparison of intravenous and intraventricular administration in beagle dogs. Neuropharmacology. 1986 Oct;25(10):1167-77. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

