Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09JXH
|
|||
| Former ID |
DIB008576
|
|||
| Drug Name |
LUM001
|
|||
| Synonyms |
SHP625
Click to Show/Hide
|
|||
| Indication | Primary biliary cholangitis [ICD-11: DB96.1] | Phase 2 | [1] | |
| Primary biliary cirrhosis [ICD-11: DB96.1; ICD-10: K74.3; ICD-9: 571.6] | Phase 2 | [2] | ||
| Primary sclerosing cholangitis [ICD-11: DB96.2; ICD-10: K83.0] | Phase 2 | [3] | ||
| Progressive familial intrahepatic cholestasis [ICD-11: 5C58.03; ICD-10: K76.8] | Phase 2 | [3] | ||
| Structural developmental anomalies of liver [ICD-11: LB20.0] | Phase 2 | [3] | ||
| Company |
Lumena pharmaceuticals
|
|||
| Structure |
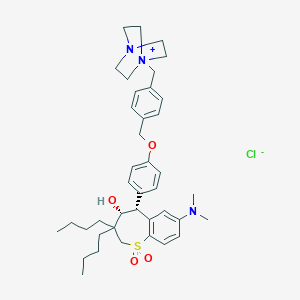 |
Download2D MOL
|
||
| Formula |
C40H56ClN3O4S
|
|||
| Canonical SMILES |
CCCCC1(CS(=O)(=O)C2=C(C=C(C=C2)N(C)C)C(C1O)C3=CC=C(C=C3)OCC4=CC=C(C=C4)C[N+]56CCN(CC5)CC6)CCCC.[Cl-]
|
|||
| InChI |
1S/C40H56N3O4S.ClH/c1-5-7-19-40(20-8-6-2)30-48(45,46)37-18-15-34(41(3)4)27-36(37)38(39(40)44)33-13-16-35(17-14-33)47-29-32-11-9-31(10-12-32)28-43-24-21-42(22-25-43)23-26-43;/h9-18,27,38-39,44H,5-8,19-26,28-30H2,1-4H3;1H/q+1;/p-1/t38-,39-;/m1./s1
|
|||
| InChIKey |
POMVPJBWDDJCMP-RUKDTIIFSA-M
|
|||
| CAS Number |
CAS 228113-66-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Ileal sodium/bile acid cotransporter (SLC10A2) | Target Info | Inhibitor | [4] |
| Sodium/bile acid cotransporter (SLC10A1) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Bile secretion | |||
| Hepatitis B | ||||
| Reactome | Recycling of bile acids and salts | |||
| WikiPathways | Bile acid and bile salt metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | ClinicalTrials.gov (NCT02321306) An Open-label Study to Evaluate the Long-term Safety and Tolerability of LUM001 in Patients With Primary Biliary Cirrhosis. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Current research on the treatment of primary sclerosing cholangitis. Intractable Rare Dis Res. 2015 Feb; 4(1): 1-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

