Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09SIR
|
|||
| Former ID |
DCL000153
|
|||
| Drug Name |
Lonidamine
|
|||
| Synonyms |
DICA; Doridamina; Lonidamin; Lonidamina; Lonidaminum; Dichlondazolic acid; Diclondazolic acid; AF 1890; L 4900; AF-1890; Doridamina (TN); KN-228; Lonidamina [INN-Spanish]; Lonidamine (INN); Lonidamine [BAN:INN]; Lonidaminum [INN-Latin]; TH-070; 1-(2,4-Dichlorbenzyl)-indazole-3-carboxylic acid; 1-(2,4-Dichlorobenzyl)-1H-indazole-3-carboxylic acid; 1-(2,4-dichlorobenzyl)indazole-3-carboxylic acid; 1-[(2,4-dichlorophenyl)methyl]-1H-indazole-3-carboxylic acid; 1-[(2,4-dichlorophenyl)methyl]indazole-3-carboxylic acid; 1H-Indazole-3-carboxylic acid, 1-((2,4-dichlorophenyl)methyl)-(9CI)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Benign prostatic hyperplasia [ICD-11: GA90; ICD-10: N40; ICD-9: 600] | Discontinued in Phase 3 | [1] | |
| Company |
Threshold Pharma
|
|||
| Structure |
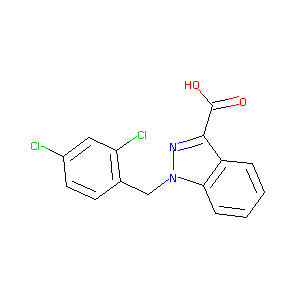 |
Download2D MOL |
||
| Formula |
C15H10Cl2N2O2
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=NN2CC3=C(C=C(C=C3)Cl)Cl)C(=O)O
|
|||
| InChI |
1S/C15H10Cl2N2O2/c16-10-6-5-9(12(17)7-10)8-19-13-4-2-1-3-11(13)14(18-19)15(20)21/h1-7H,8H2,(H,20,21)
|
|||
| InChIKey |
WDRYRZXSPDWGEB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 50264-69-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
6136961, 8176094, 11111381, 11114165, 11532966, 12012701, 14850521, 17405279, 24278515, 34705437, 47869651, 48413946, 49836722, 49857453, 50104921, 50104922, 51091595, 53777824, 53789939, 56311866, 57312316, 74608688, 80954275, 85231114, 85789040, 90341155, 91146217, 91720576, 92304137, 92716386, 104332053, 118311205, 121361741, 123093828, 124749939, 124800868, 124880555, 124880556, 124880557, 124880558, 124880559, 125351545, 126622913, 126654428, 126662083, 129566730, 134338833, 135001654, 135684247, 135692553
|
|||
| ChEBI ID |
CHEBI:50138
|
|||
| SuperDrug ATC ID |
L01XX07
|
|||
| SuperDrug CAS ID |
cas=050264692
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022059) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

