Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09TYS
|
|||
| Former ID |
DIB009291
|
|||
| Drug Name |
Loviride
|
|||
| Synonyms |
R-089439; R-89439
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Discontinued in Phase 2 | [1] | |
| Company |
Janssen Pharmaceutica NV
|
|||
| Structure |
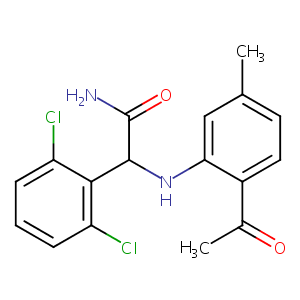 |
Download2D MOL |
||
| Formula |
C17H16Cl2N2O2
|
|||
| Canonical SMILES |
CC1=CC(=C(C=C1)C(=O)C)NC(C2=C(C=CC=C2Cl)Cl)C(=O)N
|
|||
| InChI |
1S/C17H16Cl2N2O2/c1-9-6-7-11(10(2)22)14(8-9)21-16(17(20)23)15-12(18)4-3-5-13(15)19/h3-8,16,21H,1-2H3,(H2,20,23)
|
|||
| InChIKey |
CJPLEFFCVDQQFZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 147362-57-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Reverse transcriptase (HIV RT) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002631) | |||
| REF 2 | Nucleoside reverse transcriptase inhibitor resistance mutations associated with first-line stavudine-containing antiretroviral therapy: programmatic implications for countries phasing out stavudine. J Infect Dis. 2013 Jun 15;207 Suppl 2:S70-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

