Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A0OO
|
|||
| Former ID |
DAP000170
|
|||
| Drug Name |
Amprenavir
|
|||
| Synonyms |
AMV; Agenerase; Amprenavir[usan]; Prozei; Vertex; Amprenavir [USAN]; VX 478; VX478; Vertex VX478; Agenerase (TM); Agenerase (TN); DRG-0258; GNA & Amprenavir; HHA & Amprenavir; KVX-478; VX-478; Amprenavir (JAN/USAN/INN); Tetrahydro-3-furyl N-(3-(4-amino-N-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxypropyl)carbamate; {3-[(4-AMINO-BENZENESULFONYL)-ISOBUTYL-AMINO]-1-BENZYL-2-HYDROXY-PROPYL}-CARBAMIC ACID TETRAHYDRO-FURAN-3-YL ESTER; [(3S)-oxolan-3-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate; Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl ester; Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl ester & Galanthus nivalis agglutinin (GNA); Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl ester & Hippeastrum hybrid agglutinin(HHA); (3S)-Tetrahydro-3-furanyl ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamate; (3S)-Tetrahydro-3-furyl ((alphaS)-alpha-((1R-1-hydroxy-2-(N(sup 1)-isobutylsulfanilamido)ethyl)phenethyl)carbamate; (3S)-tetrahydro-3-furyl N-[(1S,2R)-3-(4-amino-N-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxy-propyl]carbamate; (3S-(3R*(1R*,2S*)))-(3-(((4-Aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl) tetrahydro-3-furanyl carbamate; 4-Amino-N-((2 syn,3S)-2-hydroxy-4-phenyl-3-((S)-tetrahydrofuran-3-yloxycarbonylamino)-butyl)-N-isobutyl-benzenesulfonamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Approved | [1] | |
| Therapeutic Class |
Anti-HIV Agents
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
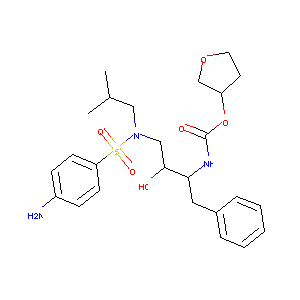 |
Download2D MOL |
||
| Formula |
C25H35N3O6S
|
|||
| Canonical SMILES |
CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2CCOC2)O)S(=O)(=O)C3=CC=C(C=C3)N
|
|||
| InChI |
1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1
|
|||
| InChIKey |
YMARZQAQMVYCKC-OEMFJLHTSA-N
|
|||
| CAS Number |
CAS 161814-49-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10286, 601727, 628182, 822019, 3727052, 3727059, 7847958, 7885327, 7978703, 8030478, 8189448, 11528777, 12014852, 14835820, 14884574, 26697364, 29215412, 43121862, 46392146, 46393211, 46507537, 53790789, 57315242, 71821412, 74965413, 85177028, 85177034, 85177055, 92309266, 93166556, 97857368, 99239834, 99239838, 99239842, 99239846, 99239847, 99239854, 99239856, 103179760, 104178998, 104253275, 104332645, 104829267, 117549941, 118048728, 125267472, 125310993, 127310203, 127310204, 127310205
|
|||
| ChEBI ID |
CHEBI:40050
|
|||
| ADReCS Drug ID | BADD_D00136 | |||
| SuperDrug ATC ID |
J05AE05
|
|||
| SuperDrug CAS ID |
cas=161814499
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Protease (HIV PR) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 2 | Darunavir: a review of its use in the management of HIV infection in adults. Drugs. 2009;69(4):477-503. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

