Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0B1VQ
|
|||
| Former ID |
DIB008785
|
|||
| Drug Name |
GR-133347
|
|||
| Synonyms |
AH-023
Click to Show/Hide
|
|||
| Indication | Anxiety disorder [ICD-11: 6B00-6B0Z; ICD-10: R45.0] | Terminated | [1] | |
| Company |
Glaxo Group Research Ltd
|
|||
| Structure |
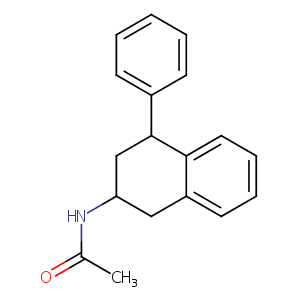 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Melatonin receptor type 1A (MTNR1A) | Target Info | Modulator | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Circadian entrainment | ||||
| Reactome | Class A/1 (Rhodopsin-like receptors) | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003420) | |||
| REF 2 | US patent application no. 5,151,446, Substituted 2-amidotetralins as melatonin agonists and antagonists. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

