Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C5VQ
|
|||
| Former ID |
DCL000199
|
|||
| Drug Name |
PRX-321
|
|||
| Synonyms |
Silybin B; Milk thistle; 142797-34-0; UNII-853OHH1429; Flavobion; Legalon 70; 853OHH1429; CCRIS 7096; Wild Artichoke; Silybum marianum; Silybin A and B; Milk thistle [NF]; Silymarin + Melatonin; AC1LU7MH; UNII-U946SH95EE; Milk Thistle (Silybum marianum) Extract or Powder; SCHEMBL751461; Silybin B, analytical standard; U946SH95EE; CHEMBL592675; Milk Thistle (Silybum marianum) Extract or Powder [seed]; DTXSID30858697; SEBFKMXJBCUCAI-WAABAYLZSA-N; MolPort-039-139-345; ZINC1530850; BDBM50088491; 2717AH; AKOS015912848
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [1] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
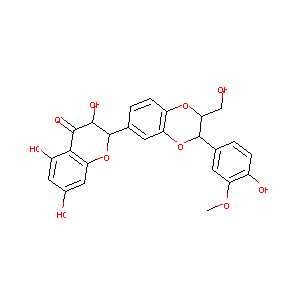 |
Download2D MOL |
||
| Formula |
C25H22O10
|
|||
| Canonical SMILES |
COC1=C(C=CC(=C1)C2C(OC3=C(O2)C=C(C=C3)C4C(C(=O)C5=C(C=C(C=C5O4)O)O)O)CO)O
|
|||
| InChI |
1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3/t20-,23-,24-,25+/m0/s1
|
|||
| InChIKey |
SEBFKMXJBCUCAI-WAABAYLZSA-N
|
|||
| CAS Number |
CAS 65666-07-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Interleukin 4 receptor alpha (IL4R) | Target Info | Agonist | [2] |
| KEGG Pathway | Cytokine-cytokine receptor interaction | |||
| PI3K-Akt signaling pathway | ||||
| Jak-STAT signaling pathway | ||||
| Hematopoietic cell lineage | ||||
| Inflammatory bowel disease (IBD) | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| IL4 Signaling Pathway | ||||
| EGFR1 Signaling Pathway | ||||
| Panther Pathway | Interleukin signaling pathway | |||
| Pathway Interaction Database | p73 transcription factor network | |||
| IL4-mediated signaling events | ||||
| WikiPathways | Inflammatory Response Pathway | |||
| IL-4 Signaling Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00797940) Convection Enhanced Localized Administration of PRX321 With Real-time Imaging for Therapy of Recurrent Glioblastoma (CLARITY-1). U.S. National Institutes of Health. | |||
| REF 2 | Convection-enhanced drug delivery of interleukin-4 Pseudomonas exotoxin (PRX321): increased distribution and magnetic resonance monitoring. J Pharmacol Exp Ther. 2009 Aug;330(2):520-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

