Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0CS2C
|
|||
| Former ID |
DCL000487
|
|||
| Drug Name |
AZD9056
|
|||
| Synonyms |
AZD-9056; AZD9056; 345304-65-6; UNII-F13K378W4L; N-(1-adamantylmethyl)-2-chloro-5-[3-(3-hydroxypropylamino)propyl]benzamide; F13K378W4L; AZD 9056; GTPL7826; SCHEMBL4126642; CHEMBL3545108; HSQAARMBHJCUOK-UHFFFAOYSA-N; MolPort-044-723-510; KS-000000WO; BCP25185; ZINC34356159; AKOS030228502; DB12594; Benzamide, 2-chloro-5-(3-((3-hydroxypropyl)amino)propyl)-N-(tricyclo(3.3.1.13,7)dec-1-ylmethyl)-; 2-Chloro-5-[3-[(3-hydroxypropyl)amino]propyl]-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)-benzamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Phase 2 | [1], [2] | |
| Company |
AstraZeneca
|
|||
| Structure |
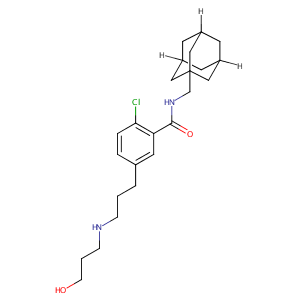 |
Download2D MOL
|
||
| Formula |
C24H35ClN2O2
|
|||
| Canonical SMILES |
C1C2CC3CC1CC(C2)(C3)CNC(=O)C4=C(C=CC(=C4)CCCNCCCO)Cl
|
|||
| InChI |
1S/C24H35ClN2O2/c25-22-5-4-17(3-1-6-26-7-2-8-28)12-21(22)23(29)27-16-24-13-18-9-19(14-24)11-20(10-18)15-24/h4-5,12,18-20,26,28H,1-3,6-11,13-16H2,(H,27,29)
|
|||
| InChIKey |
HSQAARMBHJCUOK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 345304-65-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | P2X purinoceptor 7 (P2RX7) | Target Info | Antagonist | [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Reactome | The NLRP3 inflammasome | |||
| WikiPathways | Nucleotide-binding domain, leucine rich repeat containing receptor (NLR) signaling pathways | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7826). | |||
| REF 2 | ClinicalTrials.gov (NCT00520572) A 6-month Randomised, Double-blind, Open Arm Comparator, Phase IIb, With AZD9056, in Patients With Rheumatoid Arthritis (RA). U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

