Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0D2RN
|
|||
| Former ID |
DAP001346
|
|||
| Drug Name |
Aminopterin
|
|||
| Synonyms |
APGA; Aminopteridine; Aminopterine; Aminopterinum; Aminotrexate; Pteramina; Pteramina [Czech]; A 1784; A-7170;A-Ninopterin; ENT-26079; Kyselina 4-aminolistova; Kyselina 4-aminolistova [Czech]; Kyselina 4-aminopteroylglutamova; Kyselina 4-aminopteroylglutamova [Czech]; L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]amino]benzoyl]; N-(1-((2,4-Diamino-6-pteridinylmethyl)amino)benzoyl)glutaminsaeure; N-[(4-{[(2,4-diaminopteridin-6-yl)methyl]amino}phenyl)carbonyl]glutamic acid; N-(4-{[(2,4-diaminopteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid; N-(4-(((2,4-Diamino-6-pteridinyl)methyl)amino)benzoyl)-L-glutamic acid; Kyselina N-(p-((2,4-diamino-6-pteridinyl)methyl)benzoyl)-L(+)-glutamova; Kyselina N-(p-((2,4-diamino-6-pteridinyl)methyl)benzoyl)-L(+)-glutamova [Czech]; Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)amino)benzoyl)-L(+)-glutamova; Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)amino)benzoyl)-L(+)-glutamova [Czech]; N-(4-(((2,4-Diamino-6-pteridyl)-methyl)amino)benzoyl)-L-glutamic acid; (2R)-2-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid; (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid; 2-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid; 4'-Amino-folsaeure; 4'-Desoxy-4'-aminofolsaeure; 4-Amino pteroylglutamic acid; 4-Amino-4-deoxypteroylglutamate; 4-Amino-4-desoxy-pteroylglutaminsaeure; 4-Amino-PGA; 4-Aminofolic acid; 4-Aminopteroyl-<R>glutamic acid; 4-Aminopteroyl-L-glutamic acid; 4-Aminopteroyl-glutamic acid; 4-Aminopteroylglutamic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | leukaemia [ICD-11: 2A60-2B33; ICD-9: 208.9] | Withdrawn from market | [1] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Lederle Laboratories
|
|||
| Structure |
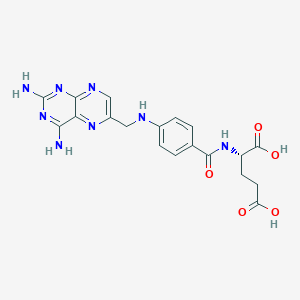 |
Download2D MOL |
||
| Formula |
C19H20N8O5
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C(=O)NC(CCC(=O)O)C(=O)O)NCC2=CN=C3C(=N2)C(=NC(=N3)N)N
|
|||
| InChI |
1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1
|
|||
| InChIKey |
TVZGACDUOSZQKY-LBPRGKRZSA-N
|
|||
| CAS Number |
CAS 54-62-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
67626, 4426032, 8151464, 11342170, 11362353, 11364803, 11367365, 11369927, 11372291, 11374552, 11378094, 11484442, 11487755, 11488709, 11491201, 11492732, 11495688, 15477678, 17404598, 24278186, 24846313, 24890768, 24890950, 26612022, 26680752, 26747419, 26747420, 26747421, 26753506, 29221333, 47365846, 47662940, 47960398, 48415548, 50105754, 50105755, 51072235, 53777140, 57264276, 57321174, 75359474, 85083722, 85083723, 85154883, 85230896, 90341578, 92124292, 92303442, 103171176, 103855303
|
|||
| ChEBI ID |
CHEBI:22526
|
|||
| SuperDrug ATC ID |
L01BA01
|
|||
| SuperDrug CAS ID |
cas=000059052
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Polypeptide deformylase (PDF) | Target Info | Inhibitor | [2], [3] |
| Pathwhiz Pathway | Folate Metabolism | |||
| Pterine Biosynthesis | ||||
| Reactome | E2F mediated regulation of DNA replication | |||
| Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation | ||||
| Metabolism of folate and pterines | ||||
| G1/S-Specific Transcription | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drug information of Aminopterin, Health Canada, 2007. | |||
| REF 2 | Loss of folylpoly-gamma-glutamate synthetase activity is a dominant mechanism of resistance to polyglutamylation-dependent novel antifolates in multiple human leukemia sublines. Int J Cancer. 2003 Feb 20;103(5):587-99. | |||
| REF 3 | vestigial suppressor genes and resistance to aminopterin in Drosophila melanogaster. Heredity. 1992 Nov;69 ( Pt 5):473-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

